3.1: Introduction
- Page ID
- 5424
\(\text{FIGURE III.1}\)
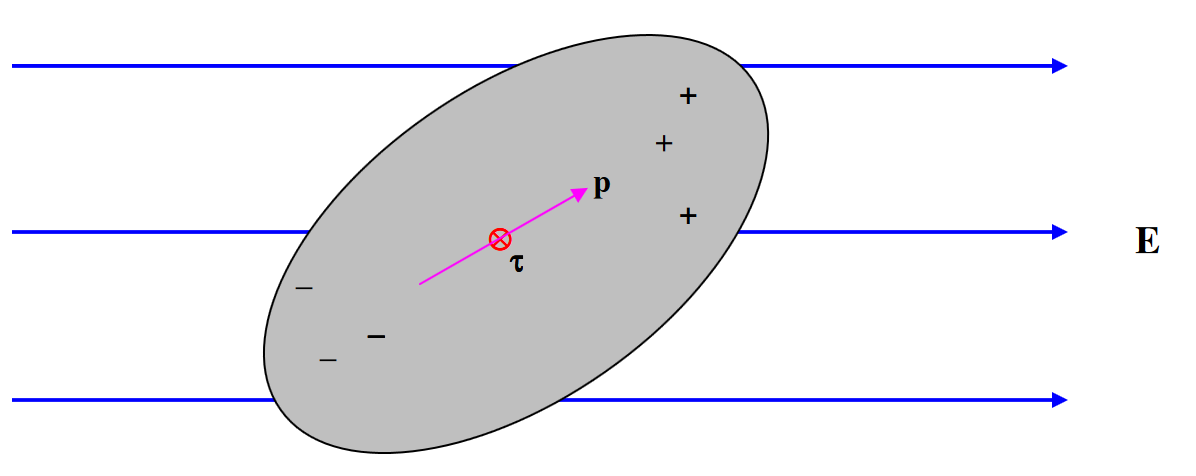
Consider a body which is on the whole electrically neutral, but in which there is a separation of charge such that there is more positive charge at one end and more negative charge at the other. Such a body is an electric dipole.
Provided that the body as a whole is electrically neutral, it will experience no force if it is placed in a uniform external electric field, but it will (unless very fortuitously oriented) experience a torque. The magnitude of the torque depends on its orientation with respect to the field, and there will be two (opposite) directions in which the torque is a maximum.
The maximum torque that the dipole experiences when placed in an external electric field is its dipole moment. This is a vector quantity, and the torque is a maximum when the dipole moment is at right angles to the electric field. At a general angle, the torque \(\tau\), the dipole moment \(\textbf{p}\) and the electric field \(\textbf{E}\) are related by
\[\tau = \textbf{p} \times \textbf{E} \tag{3.1.1} \label{3.1.1}\]
The SI units of dipole moment can be expressed as N m (V/m)-1. However, work out the dimensions of \(p\) and you will find that its dimensions are Q L. Therefore it is simpler to express the dipole moment in SI units as coulomb metre, or C m.
Other units that may be encountered for expressing dipole moment are cgs esu, debye, and atomic unit. I have also heard the dipole moment of thunderclouds expressed in kilometre coulombs. A cgs esu is a centimetre-gram-second electrostatic unit. I shall describe the cgs esu system in a later chapter; suffice it here to say that a cgs esu of dipole moment is about \(3.336 \times 10^{-12}\, \text{C m}\), and a debye (D) is \(10^{-18}\) cgs esu. An atomic unit of electric dipole moment is \(a_0e\), where \(a_0\) is the radius of the first Bohr orbit for hydrogen and \(e\) is the magnitude of the electronic charge. An atomic unit of dipole moment is about \(8.478 \times 10^{-29}\) C m.
I remark in passing that I have heard, distressingly often, some such remark as “The molecule has a dipole”. Since this sentence is not English, I do not know what it is intended to mean. It would be English to say that a molecule is a dipole or that it has a dipole moment.


