3.7: Adiabatic Processes for an Ideal Gas
( \newcommand{\kernel}{\mathrm{null}\,}\)
By the end of this section, you will be able to:
- Define adiabatic expansion of an ideal gas
- Demonstrate the qualitative difference between adiabatic and isothermal expansions
When an ideal gas is compressed adiabatically (Q=0), work is done on it and its temperature increases; in an adiabatic expansion, the gas does work and its temperature drops. Adiabatic compressions actually occur in the cylinders of a car, where the compressions of the gas-air mixture take place so quickly that there is no time for the mixture to exchange heat with its environment. Nevertheless, because work is done on the mixture during the compression, its temperature does rise significantly. In fact, the temperature increases can be so large that the mixture can explode without the addition of a spark. Such explosions, since they are not timed, make a car run poorly—it usually “knocks.” Because ignition temperature rises with the octane of gasoline, one way to overcome this problem is to use a higher-octane gasoline.
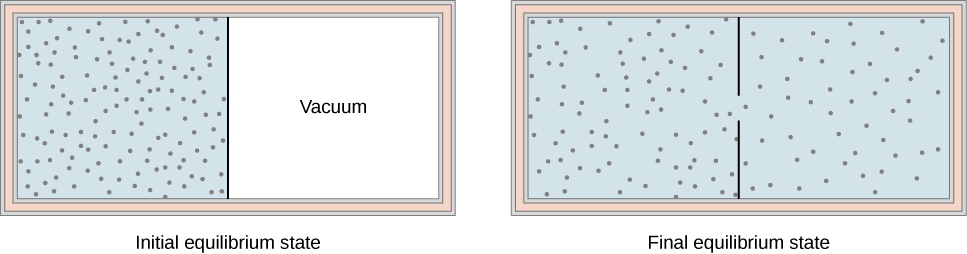
Another interesting adiabatic process is the free expansion of a gas. Figure 3.7.1 shows a gas confined by a membrane to one side of a two-compartment, thermally insulated container. When the membrane is punctured, gas rushes into the empty side of the container, thereby expanding freely. Because the gas expands “against a vacuum” (p=0), it does no work, and because the vessel is thermally insulated, the expansion is adiabatic. With Q=0 and W=0 in the first law, ΔEint=0 so Einti=Eintf for free expansion.
If the gas is ideal, the internal energy depends only on the temperature. Therefore, when an ideal gas expands freely, its temperature does not change; this is also called a Joule expansion.
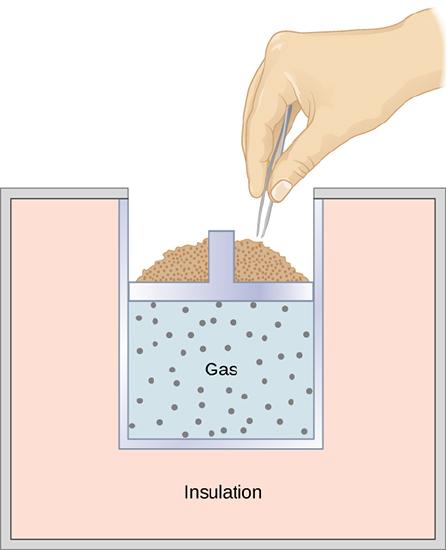
A quasi-static, adiabatic expansion of an ideal gas is represented in Figure 3.7.2, which shows an insulated cylinder that contains 1 mol of an ideal gas. The gas is made to expand quasi-statically by removing one grain of sand at a time from the top of the piston. When the gas expands by dV, the change in its temperature is dT. The work done by the gas in the expansion is dW=pdV; dQ=0 because the cylinder is insulated; and the change in the internal energy of the gas is
dEint=CVndT.
Therefore, from the first law,
CVndT=0−pdV=−pdV
so
dT=−pdVCVn.
Also, for 1 mol of an ideal gas,
[d(pV) = d(RnT), \nonumber \]
so
pdV+Vdp=RndT
and
dT=pdV+VdpRn.
We now have two equations for dT. Upon equating them, we find that
CVnVdp+(CVn+Rn)pdV=0.
Now, we divide this equation by npV and use Cp=CV+R. We are then left with
CVdpp+CpdVV=0,
which becomes
dpp+γdVV=0,
where we define γ as the ratio of the molar heat capacities:
γ=CpCV.
Thus
∫dpp+γ∫dVV=0
and
lnp+γlnV=constant.
Finally, using ln(Ax)=xlnA and lnAB=lnA+lnB, we can write this in the form
pVγ=constant.
This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process. For example, if an ideal gas makes a quasi-static adiabatic transition from a state with pressure and volume p1 and V1 to a state with p2 and V2, then it must be true that p1Vγ1=p2Vγ2.
The adiabatic condition of Equation ??? can be written in terms of other pairs of thermodynamic variables by combining it with the ideal gas law. In doing this, we find that
p1−γTγ=constant
and
TVγ−1=constant.
A reversible adiabatic expansion of an ideal gas is represented on the pV diagram of Figure 3.7.1. The slope of the curve at any point is
dpdV=ddV(constantVγ)=−γpV.
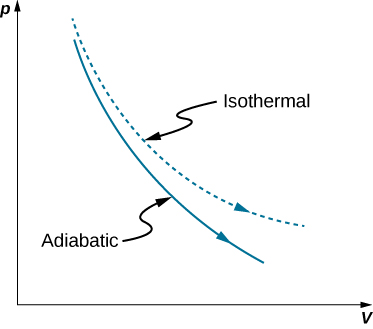
The dashed curve shown on this pV diagram represents an isothermal expansion where T (and therefore pV) is constant. The slope of this curve is useful when we consider the second law of thermodynamics in the next chapter. This slope is
dpdV=ddVnRTV=−pV.
Because γ>1, the isothermal curve is not as steep as that for the adiabatic expansion.
Gasoline vapor is injected into the cylinder of an automobile engine when the piston is in its expanded position. The temperature, pressure, and volume of the resulting gas-air mixture are 20oC, 1.00×105N/m2, and 240cm3, respectively. The mixture is then compressed adiabatically to a volume of 40cm3. Note that in the actual operation of an automobile engine, the compression is not quasi-static, although we are making that assumption here.
- What are the pressure and temperature of the mixture after the compression?
- How much work is done by the mixture during the compression?
Strategy
Because we are modeling the process as a quasi-static adiabatic compression of an ideal gas, we have pVγ=constant and pV=nRT. The work needed can then be evaluated with W=∫V2V1pdV.
Solution
- For an adiabatic compression we have p2=p1(V1V2)γ, so after the compression, the pressure of the mixture is p2=(1.00×105N/m2)(240×10−6m340×10−6m3)1.40=1.23×106N/m2. From the ideal gas law, the temperature of the mixture after the compression is T2=(p2V2p1V1)T1=(1.23×106N/m2)(40×10−6m3)(1.00×105N/m2)(240×10−6m3)⋅293K=600K=328oC.
- The work done by the mixture during the compression is W=∫V2V1pdV. With the adiabatic condition of Equation ???, we may write p as K/Vγ, where K=p1Vγ1=p2Vγ2. The work is therefore W=∫V2V1KVγdV=K1−γ(1Vγ−12−1Vγ−11)=11−γ(p2Vγ2Vγ−12−p1Vγ1Vγ−11)=11−γ(p2V2−p1V1)=11−1.40[(1.23×106N/m2)(40×10−6m3)−(1.00×105N/m2)(240×10−6m3)]=−63J.
Significance
The negative sign on the work done indicates that the piston does work on the gas-air mixture. The engine would not work if the gas-air mixture did work on the piston.


