40.4: Procedures
- Page ID
- 29351
Warnings
- Heat source and pie tins may become hot enough to cause a thermal burn.
- Always assume the heat source is hot, even when it is off.
- Excessive heating of the sugar may result in a fire alarm.
You will burn a candle and heat sugar.
-
Draw a table in which to record the products you observe in the decomposition reactions. Do not fill in data until you have read the instructions for obtaining that data.
|
Moisture |
Black Substance |
|
|---|---|---|
|
Candle + \(\ce{O2}\) |
||
|
Sugar |
Melting Wax
-
Use the aluminum foil to create a candle stand for your candle.
-
Place 3-4 ice cubes in one aluminum pie tin, and add 2 spoonful’s of water to the pie tin. Your goal is to have a little ice water in the pie tin.
-
Light the candle (\(\ce{C25H52}\)) and use the metal tongs or heat gloves to hold the pie tin with ice water, such that the pie tin is directly above the flame of the candle; the pie tin should be held about 1 centimeter above the flame. Check the bottom of the pie tin for moisture and a black substance, every few seconds. Once the moisture and the black substance appear, extinguish the candle and analyze the products you have on the bottom of the pie tin. If after 5 minutes only moisture has appeared, end the experiment. Discuss with your team what the substances look like, what they could be. Record what you think the moisture is and what you think the black substance may be, based on the elements in your reactants, in your data table.
-
Clean the bottom of the pie tin, remove all moisture and all of the black substance. Replenish the ice water in the pie tin.
Cooking Sugar
-
Set up and turn on your heat source. Ask your instructor for assistance if your team is uncertain about how to set-up a Bunsen burner.
-
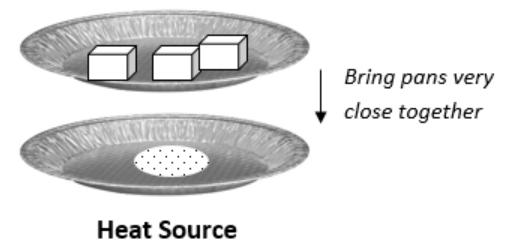
Add 1 spoonful of sugar (\(\ce{C12H22O11}\)) to the second aluminum pie tin. Spread the sugar out a little so that it is only a thin layer.
-
Use tongs and/or heat gloves to position the pie tin containing sugar in direct contact with your heat source (in contact with the flame of the Bunsen burner or directly on the hot plate). Once the sugar begins to change color and a gas is observed leaving the sugar, position the ice water pie tin 1 centimeter above the sugar pie tin, with the pie tins as close together as possible but not touching. Check the bottom of the ice water pie tin for moisture, every few seconds. Stop the experiment immediately if the sugar begins to burn (turn black). Once you see moisture on the bottom of the ice water pie tin, stop the experiment and remove the sugar from the heat source. Prolonged cooking of the sugar may set-off the fire alarm. Turn off the heat source and analyze the products. Discuss with your team what the moisture on the bottom of the ice water pie tin looks like, and what the remnants of the sugar could be. Record what you think the moisture is and what you think the remnants of the sugar may be, in your data table.
Clean-up
-
Wash and dry both pie tins – discard the sugar pie tin if it will not come clean
-
Clean your laboratory table top
-
Throw away foil used as a candle stand

