2.6: Vesicles
- Page ID
- 1354
The most basic definition of a vesicle is a compartment composed of many phospholipids with some form of head group. In a biological context, vesicles are typically formed by cells to uptake, excrete, or otherwise transport materials between membranous compartments in the cell. A synthetic vesicle, called a liposome, can be created by mixing phospholipid molecules in an aqueous environment. This article outlines some fundamental properties of vesicles, then goes on to discuss them in a biological context.
Formation of a Vesicle
Firstly, curvature must be established in a bilayer. Eventually, the indentation must protrude farther and farther, until a neck can be formed. Scission of the neck occurs to free the newly formed vesicle from the membrane. [8]

The initial indentation into the bilayer necessitates a difference in the radius of curvature between the inner and outer leaflet. Compensation for this physical requirement can happen in several ways. One method involves leaflet asymmetry, where more phospholipids are shifted to the outside leaflet to compensate for the larger radius. If different types of phospholipids with varying geometries are used, these may also be incorporated in a fashion that compensates for the difference in radii equally well. The same balance can be struck using proteins incorporated into the membrane. [8,17]
In an in vitro setting, phospholipids that are introduced to an aqueous environment can form several structures to optimize the arrangement of its hydrophobic and hydrophilic portions. Among these are micelles, liposomes, and multilamellar vesicles. Sonication of multilamellar vesicles can yield unilamellar vesicles and giant unilamellar vesicles (GUVs). Using a single type of phospholipid under these conditions yields a closed pure lipid bilayer membrane, which has been a useful model for understanding phase shifts among the lipids of the membrane, the mechanics of membrane formation and deformation, the dynamic behavior of elements that reside within the membrane, and the interactions between membranes and their environments. [2,4]
Biological Context
Vesicles mainly serve to hold some biological element apart from the surroundings. This may include biological molecules that must be transported from site to site or housing a particular chemical reaction until the products can be released into the cell.
Vesicles are most notably involved in taking nutrients in and excreting waste products out of the cell, called endocytosis and exocytosis, respectively. Vesicles serve as the chief mode of transport between the extracellular environment and various compartments in the cell such as the exosome, endosome, the trans golgi network (TGN), and the endoplasmic reticulum. Extracellular messaging works along this same system. As signals diffuse throughout the extracellular environment, they may interact with receptors that are then taken inside the cell in a vesicle or taken up inside a vesicle themselves. A number of secondary messengers may come into play to transduce the signal, or the signal may have an immediate intracellular target that elicits some change – expression of a particular gene or initiation of some post-translational modification, for example. Neuronal vesicles can carry signals between cells across the synaptic cleft or within the neuron itself.
In the cell, formation of any vesicle begins with a lipid bilayer. Perturbation and deformation at a section of this bilayer creates the proper combination of negative and positive curvature to develop the vesicle, a structure with wholly positive curvature.[8] The range of vesicles studied in a cell stretches anywhere between 30nm to 1.2µm. [6]
Vesicle Formation in vivo
The formation, or budding, of a vesicle in a cell involves deforming the membrane and creating specificity for a certain cargo. Early electron microscopy studies detected electron dense ‘coats’ in budding intermediates. The electron dense areas were later discovered to be coat proteins involved in creation of a vesicle.[10] These proteins coat a membrane section to create and stabilize curvature, then select the proper cargo. The vesicle then must be pinched off at the neck to create a membraneous structure separate from the larger membrane. These processes are typically mediated by groups of proteins. The best characterized mechanisms involve the clathrin protein, coat protein I (COPI), and coat protein II (COPII). [1, 9, 10]
 Clathrin proteins are trimers, called triskellions, best represented as a central domain composed of three heavy chains (~190kDa) interacting with each other at their C-termini, bound with three light chains (~25kDa) extending outwards. Distal to the central domain are the N-termini, seven-bladed β-propeller structures with binding sites for a multitude of adaptor proteins to transfer a variety of cargo types in the potential vesicle. In solution, triskelia form flat hexagonal rings with relative ease. For the formation of a vesicle, triskelia must also form pentagonal rings, in addition to hexagonal rings, to generate enough curvature for the encapsulation of a vesicle. At least 12 pentagons must be present in the lattice to form a full vesicle. N-terminal repeat domains of a triskelion bind different adaptor proteins that can then in turn bind cargo as well as membrane directly. A single triskelion will bind others, forming the aforementioned shapes and eventually a cage that can encapsulate a vesicle. [9,10]
Clathrin proteins are trimers, called triskellions, best represented as a central domain composed of three heavy chains (~190kDa) interacting with each other at their C-termini, bound with three light chains (~25kDa) extending outwards. Distal to the central domain are the N-termini, seven-bladed β-propeller structures with binding sites for a multitude of adaptor proteins to transfer a variety of cargo types in the potential vesicle. In solution, triskelia form flat hexagonal rings with relative ease. For the formation of a vesicle, triskelia must also form pentagonal rings, in addition to hexagonal rings, to generate enough curvature for the encapsulation of a vesicle. At least 12 pentagons must be present in the lattice to form a full vesicle. N-terminal repeat domains of a triskelion bind different adaptor proteins that can then in turn bind cargo as well as membrane directly. A single triskelion will bind others, forming the aforementioned shapes and eventually a cage that can encapsulate a vesicle. [9,10]
COPI is a heptameric protein complex associated with shuttling substrates from the cis-golgi to the rough endoplasmic reticulum, called retrograde traffic. It is composed of seven subunits (α, β, β’, δ, γ, ε and ζ), comprising the F subcomplex (a heteromer of β, δ, γ and ζ subunits) and B subcomplex (a heteromer of α and β’ subunits). The α and β’ subunits contain WD40 repeats, much like that of clathrin. COPII coat proteins are associated with the transportation of materials outward from the ER to the TGN and the cell membrane, or anterograde transfer. COPII proteins might be considered as subcomplexes of Sec13/31p and Sec23/Sec24p. Portions of the Sec13/31p have WD40 repeats, again like that of clathrin. [1,9]
Other Mechanisms of Vesicle Formation
Cytoskeletal branching, treadmilling and catastrophe affect membrane shape in the development of pseudopodia and lamellopodia, and can conceivably contribute to vesicle formation. Proteins with a BAR (Bin/amphiphysin/Rvs) domain specifically insert helices into membranes with curvature and play an important role in stabilizing curvature established by coat proteins. [8, 11, 17]
Multivesicular Bodies and Intralumenal vesicles
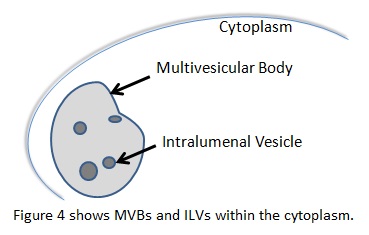 In the endocytic pathway, multivesicular bodies (MVB) are organelles that have vesicles inside their lumen, called intralumenal vesicles (ILT). They typically carry ubiquinated cargo to the lysosome, but can perform other functions as well. These vesicles require the endosomal sorting complex required for transport (ESCRT) proteins to form. ESCRTI, -II, and –III work alongside other associated proteins like vacuolar sorting protein 4 (Vsp4) to choose cargo, create a small bud, eventually perform scission on the bud, and target the vesicle to a final destination. A complex interplay between ESCRT proteins, associated targeting proteins that work with the ESCRT proteins, and post-translational modifications of the cargo governs these processes. [7] There is evidence that ESCRT proteins also participate in viral budding, cytokinesis, cell signaling, and development through its membrane interactions. [13,14,15,16]
In the endocytic pathway, multivesicular bodies (MVB) are organelles that have vesicles inside their lumen, called intralumenal vesicles (ILT). They typically carry ubiquinated cargo to the lysosome, but can perform other functions as well. These vesicles require the endosomal sorting complex required for transport (ESCRT) proteins to form. ESCRTI, -II, and –III work alongside other associated proteins like vacuolar sorting protein 4 (Vsp4) to choose cargo, create a small bud, eventually perform scission on the bud, and target the vesicle to a final destination. A complex interplay between ESCRT proteins, associated targeting proteins that work with the ESCRT proteins, and post-translational modifications of the cargo governs these processes. [7] There is evidence that ESCRT proteins also participate in viral budding, cytokinesis, cell signaling, and development through its membrane interactions. [13,14,15,16]
References
- Aridor, M., & Traub, L. M. (2002). Cargo selection in vesicular transport: the making and breaking of a coat. Traffic, 3(8), 537-546.
- Basu, S. C., & Basu, M. (Eds.). (2002). Liposome methods and protocols (No. 199). Springer Science & Business Media.
- Chenette, E. J. (2014). ESCRTing intraluminal vesicle formation. Nature cell biology, 16(5), 400-400.
- Dua, J. S., Rana, A. C., & Bhandari, A. K. (2012). Liposome: methods of preparation and applications. Int J Pharm Stud Res, 3, 14-20.
- Hanson, P. I., & Cashikar, A. (2012). Multivesicular body morphogenesis. Annual review of cell and developmental biology, 28, 337-362.
- Jena, B. P. (2008). Intracellular Organelle Dynamics at nm Resolution. Methods in cell biology, 90, 19-37.
- Jouvenet, N. (2012). Dynamics of ESCRT proteins. Cellular and Molecular Life Sciences, 69(24), 4121-4133.
- McMahon, H. T., & Gallop, J. L. (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature, 438(7068), 590-596.
- McMahon, H. T., & Mills, I. G. (2004). COP and clathrin-coated vesicle budding: different pathways, common approaches. Current opinion in cell biology, 16(4), 379-391.
- Pearse BM (April 1976). "Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles". Proceedings of the National Academy of Sciences of the United States of America 73 (4): 1255–9.
- Peter, B. J., Kent, H. M., Mills, I. G., Vallis, Y., Butler, P. J. G., Evans, P. R., & McMahon, H. T. (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science, 303(5657), 495-499.
- Piper, R. C., & Katzmann, D. J. (2007). Biogenesis and function of multivesicular bodies. Annual review of cell and developmental biology, 23, 519.
- Rusten, T. E., Vaccari, T., & Stenmark, H. (2012). Shaping development with ESCRTs. Nature Cell Biology, 14(1), 38-45.
- Slagsvold, T., Pattni, K., Malerød, L., & Stenmark, H. (2006). Endosomal and non-endosomal functions of ESCRT proteins. Trends in cell biology, 16(6), 317-326.
- Tu, C., Ahmad, G., Mohapatra, B., Bhattacharyya, S., Ortega-Cava, C., Chung, B. M., et. al & Band, H. (2011). ESCRT proteins: Double-edged regulators of cellular signaling. Bioarchitecture, 1(1), 45-48.
- Wegner, C. S., Rodahl, L. M., & Stenmark, H. (2011). ESCRT proteins and cell signalling. Traffic, 12(10), 1291-1297.
- Zimmerberg, J., & Kozlov, M. M. (2006). How proteins produce cellular membrane curvature. Nature Reviews Molecular Cell Biology, 7(1), 9-19.

