2.7: Diffusion in Membranes
- Page ID
- 1356
Eukaryotic cells are surrounded by a flexible and dynamic barrier known as a membrane. These biological membranes are composed of lipids, which aggregate to form a bilayer with particular biochemical properties. The amphipathic nature of the lipid bilayer, whose tails are hydrophobic and associate with each other and whose head groups are hydrophilic and interact with the aqueous environment, are critical to its structure. The composition of the lipid bilayer is also important for the diffusion both across and within the membrane. This membrane diffusion is important for a variety of functions, some of which include regulating the fluidity of the membrane, the uptake of metabolites into the cell from the outside, and the removal of waste products from the inside of the cell.
Fluid Mosaic Model
Each membrane protein has a particular orientation within the membrane and cannot flip-flop form one bilayer to the other after it has assumed its mature conformation. However, lateral movement within the same lipid bilayer is still possible. Lateral diffusion is a key feature of the fluid mosaic model of membrane structure that was first described in 1972 by S. Jonathan Singer and Garth Nicolson (1).
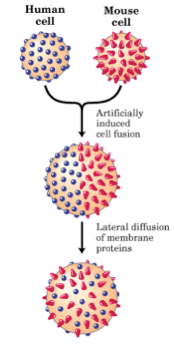
This model was supported by experiments previously done by L.D. Frye and M. Edidin in 1970, which showed that cells taken from mouse and human lines could be fused together using the Sedani virus (Figure \(\PageIndex{1}\)). The resulting fusion cell expressed both mouse and human antigens, which could be labeled indirectly by fluorescent antibodies and followed. Mixing of both parental antigens occurred forty minutes after fusion, suggesting that lateral diffusion within the membrane can occur (2). However, the time that it takes for lateral diffusion to occur depends on membrane fluidity, which ultimately depends on both temperature and lipid composition.
Types of Diffusion Across the Plasma Membrane
There are general thermodynamic principles that govern the transfer of molecules across the membrane. Figure \(\PageIndex{2}\) represents the equation that shows the amount of free energy that is required for a substrate to cross a membrane. In order for diffusion to occur, \(\Delta G\) must be negative and as \(\Delta G\) moves away from zero and becomes more positive, work becomes required. When the concentrations become equal on both sides of the membrane and \(\Delta G=0\) and the rates of transport in both directions will be the same and no net transport will occur.
\[ \Delta G =RT \ln \left( \dfrac{C_2}{C_1}\right) \]
If C2, the concentration of a substrate in the cytosol, is less than C1, the concentration of a substrate outside of the cell, then \(\Delta G\) is negative and the process is favorable. Gradually, as more substrate is transferred across the membrane, C1 decreases as C2 increases until C2 = C1and at this point delta G =0 and the system is at equilibrium.
Simple Diffusion
Simple diffusion occurs by the diffusion of molecules, such as O2 and CO2, across the hydrophobic core of the membrane (Figure \(\PageIndex{3}\)). Therefore, no ATP is required for this type of diffusion across the membrane, it is simply a matter of molecules moving down a concentration gradient. As simple diffusion does not require ATP, large polar molecules or ions cannot diffuse across the membrane. This is due to the hydrophobic tail region of the membrane, which presents too large of an energy barrier to be overcome by the potential stored in the gradient.
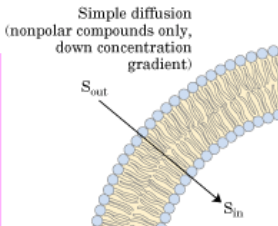
The net rate of transport is proportional to the concentration difference (C2 – C1) across the membrane (Figure \(\PageIndex{4}\)).
\[ J = - \dfrac{KD_1(C_2-C_1)}{l}\]
where
- \(J\) is the net rate of transport,
- \(K\) is the partition coefficient for the ratio of solubilities of the material in lipid and water,
- \(D_1\) is the diffusion coefficient of the diffusing substance in the membrane, and
- \(l\) is the thickness of the membrane.
For ions and other hydrophilic substances, K is a very small number, given that diffusion of such molecules across the membrane is very slow.
Facilitated Transport
As opposed to simple diffusion, which does not require ATP, facilitated transport needs ATP in order to overcome the energy barrier of the hydrophobic tail region of the membrane. In addition, this type of diffusion is dependent on cargo binding the membrane-embedded channel or carrier protein.There are two types of facilitated transport, pore-facilitated transport and carrier-facilitated transport. In order to distinguish between pore-facilitated transport and carrier-facilitated diffusion, one can fluctuate the membrane fluidity by altering the temperature. This change in temperature will stop carrier-facilitated diffusion due to the fact that carrier-facilitated diffusion must move through the membrane in order to function and cannot do so when the membrane is in a non-fluid state.
Pore-Facilitated Transport
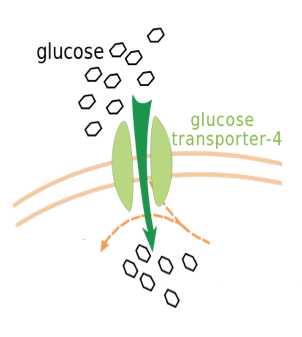
Pore-facilitated transport uses proteins embedded within the membrane that can open and close in order to facilitate diffusion. This type of diffusion allows for selected ions to pass through the pore, such as Cl-. An important example of pore-facilitated transport is the transport of glucose through the use of a gated pore mechanism, in which the pore is never open at both ends at once (Figure \(\PageIndex{5}\)). Instead, the pore opens at the exterior to allow the entry of glucose, closes the exterior opening, opens the interior opening, releases glucose into the cytosol, and finally returns to its state of binding on the exterior (15).
Carrier-Facilitated Transport
Antibiotic ionophores, such as valinomycin, a cation carrier, are an example of carrier-facilitated transport. Its folded conformation allows for the protein to have an outer hydrophobic surface, making it soluble in the lipid bilayer, with an internal conformation that mimics the hydration shell that the cation would have in an aqueous solution. This conformation allows valinomycin to diffuse from one surface of a membrane, pick up an ion, and then diffuse to the other surface to release it (Figure \(\PageIndex{6}\)).

Factors that Influence Membrane Diffusion
There are several factors that can influence the diffusion of molecules both within and across a membrane; physical barriers, electrostatic attraction or repulsion nodes, and partitioning phenomena (3), are just a few examples.
Physical Obstacles
Physical obstacles can become considerably crowded, which can obstruct the free passage of molecules. Several adaptor proteins (alpha-actinin, talin, vinculin, etc.) can attach the cortical cytoskeleton to the long cytosolic tails of transmembrane proteins within the plasma membrane and act as a fence, which restricts the movement of molecules across the membrane (4,5). Such obstruction by the cortical cytoskeleton has lead to the observation that molecules undergo a “hop diffusion” in which they “hop” intermittently between confinement zones (4,5) in order to diffuse (Figure7).

Membrane-matrix junctions can also contribute to a physical obstruction that impedes membrane diffusion. This is due to the binding between integrin receptors located in the plasma membrane and the extracellular matrix (fibrous network of proteins that cells attach to) (Figure \(\PageIndex{8}\)). If this interaction is increasingly accumulated at a high enough density, then diffusion is limited. A recent study showed that this type of physical barrier blocked the diffusion of membrane molecules whose dimensions exceeded the width of the integrin-matrix interaction (6).

Electrostatic Impediments
Electrostatic interactions can also interfere with free diffusion, as charged proteins or lipids can be repelled by like charges or attracted by opposite ones (Figure \(\PageIndex{9}\)). McLaughlin and Murray in 2005 showed that proteins have natively unfolded regions that have both basic and hydrophobic residues that allow them to exist within the bilayer and at the same time attract anionic lipids electrostatically (7). As a result, the membrane-associated cationic residues associate together, creating a negatively charged ring around the protein. Thus, the resulting ring of anionic lipids can alter the mobility of other charged molecules in the plane of the membrane (8).
Partition-Induced Barriers
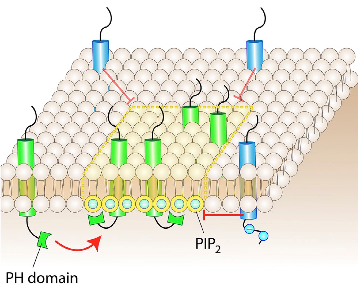
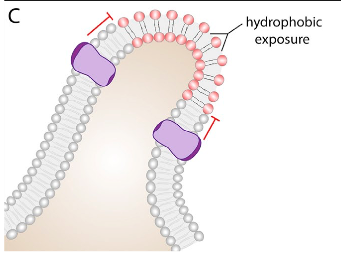
In a membrane, certain types of lipids or proteins can partition into defined regions, resulting in an area of subdiffusive behavior (3). The association between lipids and proteins is often driven by the recognition of particular binding domains, for example, the association of protein PH domains with phosphoinositides (9). Another way for these lipid-protein complexes to form is through hydrophobic interactions. An example of this hydrophobic interaction-driven complex is the saturated lipid- and cholesterol- rich microdomains, known as lipid rafts (10). Lastly, membrane curvature can play a role in the obstruction of diffusion (Figure10). The bent region of the membrane has distinct properties, as the head groups of the lipids constituting the concave monolayer are unusually close, whereas the head groups on the convex monolayer are uncharacteristically far apart. The concave side of the bent bilayer can alter diffusion physically or electrostatically, while the convex side creates a more accessible membrane due to reduced packing of the head groups.
Techniques to Monitor Diffusion
- Fluorescence recovery after photobleaching (FRAP) was initially very useful in studying the lateral diffusion of membrane components (11). This technique uses fluorescently labeled probes to follow a molecule of interest. By using a high intensity laser, the fluorophores in a region of interest will become bleached and lose signal (Figure11). The probes that were not bleached will then diffuse throughout the sample and replace the bleached region. Since this method measures the average behavior of molecule, it ultimately has limited temporal resolution (seconds).
- Fluorescence correlation spectroscopy (FCS) is a correlation analysis that measures fluctuation in fluorescence intensity. This technique provides high spatial precision and can measure diffusion coefficients of molecules (12,13). Unlike FRAP, FCS can acquire the time resolution, but lacks the ability to capture defined transient events.
- Imaging total internal reflection (ITIR)-FCS is a technique that can circumvent both of the problems in other techniques, as it can probe diffusion in membranes with good temporal and spatial resolution (12). ITIR-FCS can be applied to diffusion, active transport, or even both.
References
- Singer S.J. and Nicolson G.L. 1972. The Fluid Mosaic Model of the Structure of Cell Membranes. Science. 175:720-31.
- Frye L.D. and Edidin M. 1970. The Rapid Intermixing of Cell Surface Antigens After Formation of Mouse-Human Heterokaryons. Journal of Cell Science. 7:319-35.
- Mathews C.K. and Van Holde K.E. 1996. Biochemistry. Second Edition. Menlo Park, CA: The Benjamin/Cummings Publishing Company, Inc.
- Fujiwara T.K., Ritchie H., Murakoshi K., Jacobson, Kusumi 2002. Phospholipids undergo hop diffusion in compartmentalized cell membrane. Journal of Cell Biology. 157:1071-82.
- Suzuki K.G., Fujiwara T.K., Sanematsu F., Iino R., Edidin M., Kusumi A 2007. GPI-anchored receptor clusters transiently recruit Lyn and G-alpha for temporary cluster immobilization and Lyn activation: single molecule tracking study. Journal of Cell Biology. 177:717-30.
- Paszek M.J., DuFort C.C., Rossier O., Bainer R., Mouw J.K., Godula K., Hudak J.N., Lakins A.C., Wijekoon L., Cassereau 2014. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 511:319-25.
- McLaughlin S. and Murray D. 2005. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 438:605-611.
- Van den Boggart G., Meyenberg K., Risselada H.J., Amin K.I., Willig B.E., Hubrich M., Dier S.W. Hell, H., Grubmuller U., Diederichsen, Jahn R., 2011. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 479: 552-55.
- Trimble W.S. and Grinstein S. 2015. Barriers to the free diffusion of proteins and lipids in the plasma membrane. Journal of Cell Biology. 208:259- 71
- Lemmon M.A. 2008. Membrane recognition by phospholipid-binding domains. Nature Review Molecular and Cell Biology. 9: 99-111.
- Lingwood D. and Simons K., 2010. Lipid rafts as a membrane-organizing principle. Science. 327: 46-50.
- Chen Y., Lagerholm B.C., Yang B., Jacobson K., 2006. Methods to measure the lateral diffusion of membrane lipids and proteins. Methods. 39:147-53.
- Sankaran J., Manna M., Guo L., Kraut R., Wohland T. 2009. Diffusion, transport, and cell membrane organization investigated by image fluorescence cross-correlation spectroscopy. Biophysical Journal. 97:2630-39.
- Magde D., Elson E.L., Webb W.W. 1974. Fluorescence correlation spectroscopy. Biopolymers. 17:361-76.
- Oka Y., Asaon T., Shibasaki Y., Lin J.L., Tsukuda K., Katagiri H., Akanuma Y., Takaku F. 1990. C-terminal truncated glucose transporter is locked into an inward-facing form without transport activity. Nature. 345:550-53.

