4: The Third Law of Thermodynamics
( \newcommand{\kernel}{\mathrm{null}\,}\)
The second law defines entropy by the relation dQ=TdS. Thus entropy is defined only up to an additive constant. Further, it does not tell us about the behavior of S as a function of T close to absolute zero. This is done by the third law.
The contribution to the entropy by each set of degrees of freedom in internal thermodynamic equilibrium tends to zero in a differentiable way as the absolute zero of temperature is approached.
The limiting value of S is independent of the process by which T=0 is approached; it does not matter whether the system is in liquid or solid phase, whether it is under pressure, etc. Further, the differentiability condition says that (∂S∂T) is finite at absolute zero, where the derivative is taken along any process.
Unattainability of Absolute Zero
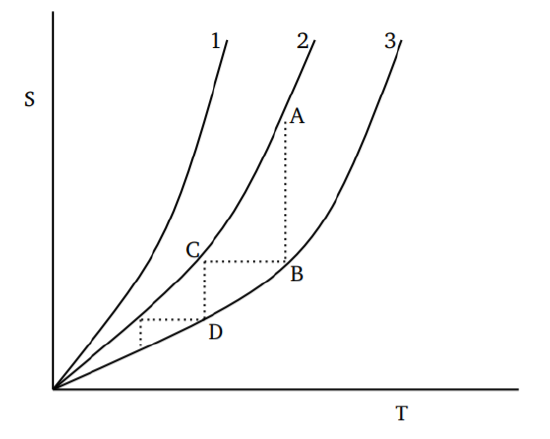
For different starting points, the variation of entropy with temperature will be as shown in Figure 4.1. A process that reduces the temperature can be viewed as an isothermal decrease of entropy from A to B, followed by an isentropic (adiabatic) decrease of temperature along BC. By continuing the process, one can get closer and closer to absolute zero. But it is obvious that the temperature decreases achieved will become smaller and smaller; after any finite number of steps, there will still be a positive nonzero temperature. Thus we conclude that only an asymptotic approach to absolute zero is possible for any thermodynamic process. Since any thermodynamic process for cooling can be built up as a sum of steps like ABC, the conclusion holds in general.
Vanishing of Specific Heats at Absolute Zero
The specific heat for any process can be written as
CR=(∂Q∂T)R=T(∂S∂T)R
where R specifies the quantity held constant. Since, by the third law, (∂Q∂T)R is finite as T→0, we find CR→0 as T→0. In particular, the specific heats at constant volume (Cv) and at constant pressure (Cp) vanish at absolute zero.


