3.4: The Gel Phase
- Page ID
- 1357
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The lipid bilayer is a thermodynamic system that transitions from the relatively disordered liquid phase to the relatively ordered gel or solid-phase when enough heat is removed from the system [1]. In this page we will detail the gel phase of the lipid bilayer. Biological plasma membranes express a plethora of unique lipids, sterols, and proteins and exhibit greater complexity than model lipid bilayer systems. At temperatures above the melting point (Tm) the lipid bilayer exists in the liquid phase while at temperatures below Tm, the lipid bilayer enters the gel phase. In the gel phase, the lipid bilayer undergoes a fundamental molecular reorganization characterized by an increase in order, a loss of lateral mobility within a given leaf of the bilayer as well as a change in acyl chain configuration within a single lipid molecule, and a change in the broad topology of the membrane [1, 2, 3].
Review of lipid bilayer phases and phase transitions
The lipid bilayer is a thermodynamic system that exists in phases. These phases are largely characterized by the relative lateral mobility of lipids within the bilayer, the molecular conformation of lipids within the bilayer, and the topological structure of the bilayer. Lipid bilayers exist in either the liquid or gel phase, at a broad range of temperatures. These phases are separated by the temperature at which the bilayer will undergo the transition from one phase to the other, termed \(T_m\). \(T_m\) is impacted by many factors e.g. acyl chain length and saturation state, the ionic conditions of the solvent, presence of sterols. At temperatures above Tm, the lipid bilayer exists in the liquid phase, characterized by a relatively high degree of lateral mobility of individual lipids within the bilayer. At temperatures below \(T_m\), the lipid bilayer exists in the gel or solid phase [1, 2, 3]. The gel phase is described in detail below.
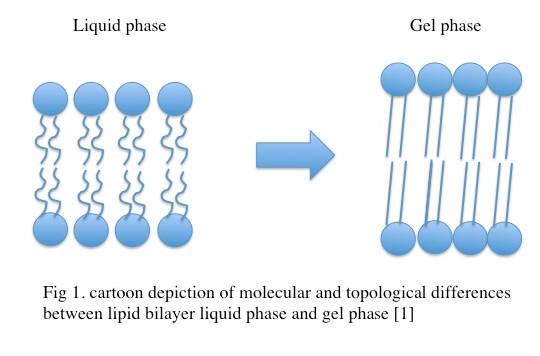
Molecular characterization of the gel phase
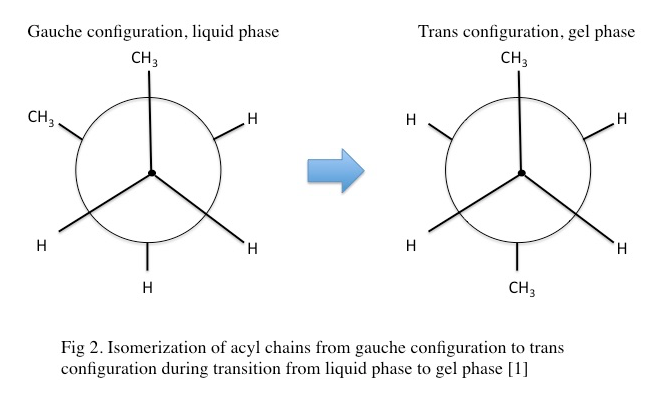
In the gel phase, individual lipids within the lipid bilayer lose lateral mobility, associated with an increase in order. The lipid head groups become more tightly packed and dehydrated, and the lipid acyl chain tails become fully extended and more tightly packed with increased van der waals interactions as seen in Figure \(\PageIndex{1}\). During the phase transition from the liquid phase to the gel phase, the acyl chain tails undergo an isomerization from a gauche conformation to a trans conformation. That is, in the gel phase, the acyl chains contain relatively few gauche conformations and are in the trans conformation. This change in conformation to mostly trans is thought to promote the tighter packing arrangements as it removes “kinks” in the acyl chain present in the lipid phase. In the gel phase, the lipid bilayer also becomes thicker relative to the liquid phase as seen in Figure \(\PageIndex{2}\) [1] . In the gel phase, the lipid bilayer also increases in stiffness than in the liquid phase. The stiffness of lipid bilayers in the liquid phase is typically ~\(10\m k_BT\) (where \(k_B\) is the Boltzmann constant
multiplied by the temperature) while the stiffness of lipid bilayers in the gel phase are 30-50 kBT [4, 7]. This increase fundamentally impacts the propensity of the lipid bilayer to curve and bend as well as the inherent fluctuations of the lipid bilayer [methods in membrane lipids]. As previously mentioned, the length of the lipid acyl chains impacts Tm. As an example, 1,2-dilauroyl-sn-phosphatidylcholine (DLPC) with an acyl chain length of 12 carbons is in the liquid phase at room temperature while 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) with an acyl chain length of 18 carbons is in the gel phase at room temperature [1]. The structure of gel phase dipalmitoylphosphatidylcholine (DMPC) has also been investigated by x-ray diffractions providing insight into the topological organization of the gel phase [6]. The structure of gel phase DPPC has also been investigated using similar methodology [10]. The topological organization of gel phase phosphatidylethanolamine has been studied using neutron diffraction [11].
Biophysical characterization of the gel phase
At temperatures below Tm, the lipid bilayer undergoes a fundamental reorganization to occupy the lowest free energy state. This fundamental reorganization is the phase transition from the liquid phase to the gel phase. This is a first order phase transition characterized by a discontinuous change in density [1]. As an example, a dipalmitoylphosphatidylcholine (DPPC) lipid bilayer enters the gel phase at temperatures below 15 °C [4].
The transition between phases has been experimentally investigated using techniques such as scanning calorimetry and fourier transformed infrared (FTIR) spectroscopy. FTIR is a spectroscopic method that measures the vibrational frequency of the ethyl groups on the acyl tails. As such, in FTIR, lipid bilayers in the gel phase display low vibrational frequency that increases as a function of temperature [1]. The transition to the gel phase from the liquid phase has been described by the nucleation and growth theory; in which a critical nucleus is formed that then serves as the initiation event for lateral formation of the gel lipid bilayer. The formation of the critical nucleus within the liquid lipid bilayer has an associated free energy described by equation 1:
\[ ΔG=Δμn+2γ[πσn]1/2 \tag{1}\]
Where Δμ is the chemical potential of gel phase lipids, n is the number of lipids in the critical nucleus, γ is the line tension between the phases, and σ is the area per lipid in the gel phase. This nucleation and growth event has been modeled in molecular dynamics simulations [5, 9].
The gel phase in more complex lipid bilayers
Lipid bilayers composed of different lipid types exhibit more complex behavior than simple lipid bilayers composed of a single lipid species. The addition of a second lipid species, with a different associated Tm to a bilayer impacts the transition to the gel phase by changing the Tm of the system. The global behavior of this complex lipid bilayer is largely dependent on the abundance of each lipid species such that a threshold population of each lipid species is necessary for cooperative phase separation [2]. Phase separation has been observed in lipid bilayers composed of mixed lipid species and has been modeled in molecular dynamics simulations as seen in Figure \(\PageIndex{3}\) [1]. Also, phase separation in which one lipid species resides in the liquid phase and the other lipid species resides in the solid (gel) phase can also occur. A predicted Tm for these mixed lipid bilayers can be calculated although it is important to note that lipid species with higher Tms have greater enthalpy and thus contribute more to the Tm in a mixed species bilayer, thus making the predicted Tm deviate from the actual Tm [1].
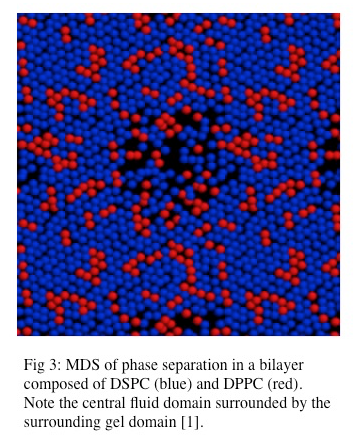
While it is generally accepted that biological lipid bilayers do not largely exist in the gel phase, simple phase coexistence in lipid bilayers has been observed. This organization has been described as liquid-ordered, in which lipid domains with tight packing but high rates of lateral mobility exist within the larger area of liquid-disordered lipids [1, 2, 3, 12]. However our understanding of the gel phase provides direct insight into our understanding of these lipid microdomains. It is also clear that phase transition in biological membranes results in packing defects and results in “leakiness” during the phase transition [1]. Phase separation has been investigated using atomic force microscopy. These experiments have demonstrated that lipid domains with unique topological character do exist within lipid bilayers [8].
Concluding Remarks
The lipid bilayer is a thermodynamic system. Like other thermodynamic systems, the lipid bilayer exists in phases. At temperatures below the Tm of the lipid bilayer, the lipid bilayer enters the gel phase. The gel phase is characterized by a high degree of order, decreased lateral mobility of lipids within a leaf of the bilayer, tight packing, acyl chain straightening (due to isomerization of the acyl chains to mostly trans state), and thickening of the bilayer relative to the liquid phase [1, 2, 3].
References
- Faller, R. MCB241 Lectures (2015)
- Mukherjee, S. and F. R. Maxfield (2004). "Membrane domains." Annu Rev Cell Dev Biol 20: 839-866.
- van Meer, G., et al. (2008). "Membrane lipids: where they are and how they behave." Nat Rev Mol Cell Biol 9(2): 112-124.
- Dopico, Alex. Methods in membrane lipids. Humana, 2007. Print.
- Marrink, S. J., et al. (2005). "Simulation of gel phase formation and melting in lipid bilayers using a coarse grained model." Chem Phys Lipids 135(2): 223-244.
- Tristram-Nagle, S., et al. (2002). "structure of gel phase DMPC determined by x-ray diffraction." Biophys J 83.
- Picas, L., et al. (2012). "Direct measurement of the mechanical properties of lipid phases in supported bilayers." Biophys J 102(1): L01-03.
- Goksu, E. I., et al. (2009). "AFM for structure and dynamics of biomembranes." Biochim Biophys Acta 1788(1): 254-266.
- Heller, H., et al. (1993). "molecular dynamics simulation of a bilayer of 200 lipids in the gel and in the liquid crystal phases " Journal of physical chemistry 97.
- Wiener, M. C., et al. (1989). "structure of the fully hydrated gel phase of DPPC." Biophys J 55.
- Builtd, G. and J. Seeleg (1980). "conformation of phosphatidylethanolamine in the gel phase as seen by neutron diffraction." Biochemistry 19.
- Schram, V., et al. (1996). "topology of gel phase domains and lipid mixing properties in phase separated two-component phosphatidylcholine bilayers." Biophys J 71.

