23.1: The Electromagnetic Spectrum
- Page ID
- 15660
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)learning objectives
- Compare properties of AM and FM radio waves
Radio Waves
Radio waves are a type of electromagnetic (EM) radiation with wavelengths in the electromagnetic spectrum longer than infrared light. They have have frequencies from 300 GHz to as low as 3 kHz, and corresponding wavelengths from 1 millimeter to 100 kilometers. Like all other electromagnetic waves, radio waves travel at the speed of light. Naturally occurring radio waves are made by lightning or by astronomical objects. Artificially generated radio waves are used for fixed and mobile radio communication, broadcasting, radar and other navigation systems, communications satellites, computer networks and innumerable other applications. Different frequencies of radio waves have different propagation characteristics in the Earth’s atmosphere—long waves may cover a part of the Earth very consistently, shorter waves can reflect off the ionosphere and travel around the world, and much shorter wavelengths bend or reflect very little and travel on a line of sight.

Electromagnetic Spectrum: The electromagnetic spectrum, showing the major categories of electromagnetic waves. The range of frequencies and wavelengths is remarkable. The dividing line between some categories is distinct, whereas other categories overlap. Microwaves encompass the high frequency portion of the radio section of the EM spectrum.
Types of Radio Waves and Applications
Radio waves have many uses—the category is divided into many subcategories, including microwaves and electromagnetic waves used for AM and FM radio, cellular telephones and TV.
The lowest commonly encountered radio frequencies are produced by high-voltage AC power transmission lines at frequencies of 50 or 60 Hz. These extremely long wavelength electromagnetic waves (about 6000 km) are one means of energy loss in long-distance power transmission.
Extremely low frequency (ELF) radio waves of about 1 kHz are used to communicate with submerged submarines. The ability of radio waves to penetrate salt water is related to their wavelength (much like ultrasound penetrating tissue)—the longer the wavelength, the farther they penetrate. Since salt water is a good conductor, radio waves are strongly absorbed by it; very long wavelengths are needed to reach a submarine under the surface.
AM Radio Waves
AM radio waves are used to carry commercial radio signals in the frequency range from 540 to 1600 kHz. The abbreviation AM stands for amplitude modulation—the method for placing information on these waves. A carrier wave having the basic frequency of the radio station (for instance, 1530 kHz) is varied or modulated in amplitude by an audio signal. The resulting wave has a constant frequency, but a varying amplitude.
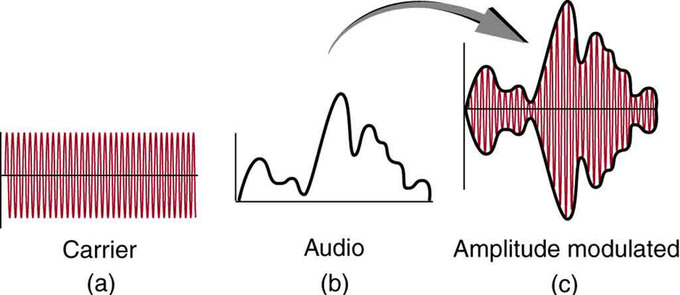
AM Radio: Amplitude modulation for AM radio. (a) A carrier wave at the station’s basic frequency. (b) An audio signal at much lower audible frequencies. (c) The amplitude of the carrier is modulated by the audio signal without changing its basic frequency.
FM Radio Waves
FM radio waves are also used for commercial radio transmission, but in the frequency range of 88 to 108 MHz. FM stands for frequency modulation, another method of carrying information. In this case, a carrier wave having the basic frequency of the radio station (perhaps 105.1 MHz) is modulated in frequency by the audio signal, producing a wave of constant amplitude but varying frequency.
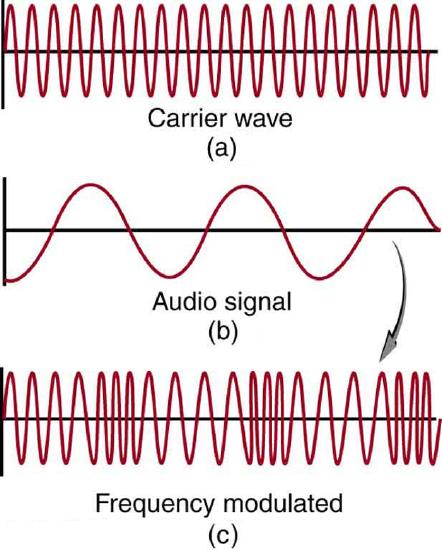
FM Radio: Frequency modulation for FM radio. (a) A carrier wave at the station’s basic frequency. (b) An audio signal at much lower audible frequencies. (c) The frequency of the carrier is modulated by the audio signal without changing its amplitude.
Since audible frequencies range up to 20 kHz (or 0.020 MHz) at most, the frequency of the FM radio wave can vary from the carrier by as much as 0.020 MHz. For this reason, the carrier frequencies of two different radio stations cannot be closer than 0.020 MHz. An FM receiver is tuned to resonate at the carrier frequency and has circuitry that responds to variations in frequency, reproducing the audio information.
FM radio is inherently less subject to noise from stray radio sources than AM radio because amplitudes of waves add noise. Thus, an AM receiver would interpret noise added onto the amplitude of its carrier wave as part of the information. An FM receiver can be fashioned to reject amplitudes other than that of the basic carrier wave and only look for variations in frequency. Thus, since noise produces a variation in amplitude, it is easier to reject noise from FM.
TV
Electromagnetic waves also broadcast television transmission. However, as the waves must carry a great deal of visual as well as audio information, each channel requires a larger range of frequencies than simple radio transmission. TV channels utilize frequencies in the range of 54 to 88 MHz and 174 to 222 MHz (the entire FM radio band lies between channels 88 MHz and 174 MHz). These TV channels are called VHF (very high frequency). Other channels called UHF (ultra high frequency) utilize an even higher frequency range of 470 to 1000 MHz.
The TV video signal is AM, while the TV audio is FM. Note that these frequencies are those of free transmission with the user utilizing an old-fashioned roof antenna. Satellite dishes and cable transmission of TV occurs at significantly higher frequencies, and is rapidly evolving with the use of the high-definition or HD format.
Microwaves
Microwaves are electromagnetic waves with wavelengths ranging from one meter to one millimeter (frequencies between 300 MHz and 300 GHz).
Learning objectives
- Distinguish three ranges of the microwave portion of the electromagnetic spectrum
Microwaves
Microwaves are electromagnetic waves with wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently with frequencies between 300 MHz (0.3 GHz) and 300 GHz. The microwave region of the electromagnetic (EM) spectrum is generally considered to overlap with the highest frequency (shortest wavelength) radio waves. As is the case for all EM waves, microwaves travel in a vacuum at the speed of light. The prefix “micro-” in “microwave” is not meant to suggest a wavelength in the micrometer range. It indicates that microwaves are “small” because have shorter wavelengths as compared to waves used in typical radio broadcasting. The boundaries between far infrared light, terahertz radiation, microwaves, and ultra-high-frequency radio waves are fairly arbitrary. They are used variously between different fields of study (see figure).
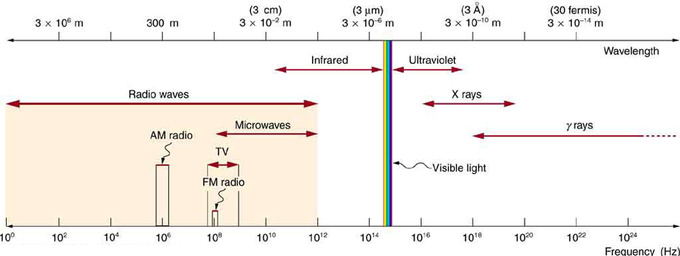
Electromagnetic Spectrum: The electromagnetic spectrum, showing the major categories of electromagnetic waves. The range of frequencies and wavelengths is remarkable. The dividing line between some categories is distinct, whereas other categories overlap. Microwaves overlap with the high frequency portion of the radio section of the EM spectrum.
Subcategories of Microwaves
The microwave portion of the radio spectrum can be subdivided into three ranges, listed below from high to low frequencies.
- Extremely high frequency (EHF) is the highest microwave frequency band. EHF runs the range of frequencies from 30 to 300 gigahertz, above which electromagnetic radiation is considered as far infrared light, also referred to as terahertz radiation. This frequency range corresponds to a wavelength range of 10 to 1 millimeter, so it is sometimes called the millimeter band. This band is commonly used in radio astronomy and remote sensing.
- Super high frequency (SHF) is the designation for electromagnetic wave frequencies in the range of 3 GHz to 30 GHz. This band of frequencies is known also as the centimeter band because the wavelengths range from ten to one centimeters. This frequency range is used for most radar transmitters, microwave ovens, wireless LANs, cell phones, satellite communication, microwave radio relay links, and numerous short range terrestrial data links.
- Ultra-high frequency (UHF) designates the microwave frequency range of electromagnetic waves between 300 MHz and 3 GHz, also known as the decimeter band because the wavelengths range from one to ten decimeters, or 10 centimeters to 1 meter. They are used for television broadcasting, cordless phones, walkie-talkies, satellite communication, and numerous other applications.
Sources of Microwaves
Microwaves are the highest-frequency electromagnetic waves that can be produced by currents in macroscopic circuits and devices. Microwaves can also be produced by atoms and molecules—e.g., they are a component of electromagnetic radiation generated by thermal agitation. The thermal motion of atoms and molecules in any object at a temperature above absolute zero causes them to emit and absorb radiation.
Since it is possible to carry more information per unit time on high frequencies, microwaves are quite suitable for communications devices. Most satellite-transmitted information is carried on microwaves, as are land-based long-distance transmissions. A clear line of sight between transmitter and receiver is needed because of the short wavelengths involved.
The sun also emits microwave radiation, although most of it is blocked by Earth’s atmosphere. The Cosmic Microwave Background Radiation (CMBR) is microwave radiation that permeates all of space, and its discovery supports the Big Bang theory of the origin of the universe.
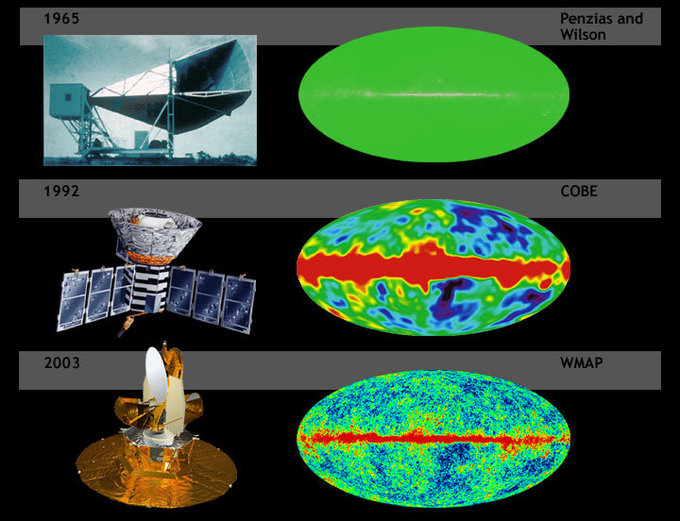
Cosmic Microwave Background: Cosmic background radiation of the Big Bang mapped with increasing resolution.
Devices Employing Microwaves
High-power microwave sources use specialized vacuum tubes to generate microwaves. These devices operate on different principles from low-frequency vacuum tubes, using the ballistic motion of electrons in a vacuum under the influence of controlling electric or magnetic fields, and include the magnetron (used in microwave ovens), klystron, traveling-wave tube (TWT), and gyrotron.

Cavity Magnetron: Cutaway view inside a cavity magnetron as used in a microwave oven.
Microwaves are used by microwave ovens to heat food. Microwaves at a frequency of 2.45 GHz are produced by accelerating electrons. The microwaves then induce an alternating electric field in the oven. Water and some other constituents of food have a slightly negative charge at one end and a slightly positive charge at one end (called polar molecules). The range of microwave frequencies is specially selected so that the polar molecules, in trying to maintain their orientation with the electric field, absorb these energies and increase their temperatures—a process called dielectric heating.
Radar, first developed in World War II, is a common application of microwaves. By detecting and timing microwave echoes, radar systems can determine the distance to objects as diverse as clouds and aircraft. A Doppler shift in the radar echo can determine the speed of a car or the intensity of a rainstorm. Sophisticated radar systems can map the Earth and other planets, with a resolution limited by wavelength. The shorter the wavelength of any probe, the smaller the detail it is possible to observe.
A maser is a device similar to a laser, which amplifies light energy by stimulating photons. The maser, rather than amplifying visible light energy, amplifies the lower-frequency, longer-wavelength microwaves and radio frequency emissions.
Infrared Waves
Infrared (IR) light is EM radiation with wavelengths longer than those of visible light from 0.74 µm to 1 mm (300 GHz to 1 THz).
Skills to Develop
- Distinguish three ranges of the infrared portion of the spectrum, and describe processes of absorption and emission of infrared light by molecules
Infrared Waves
Infrared (IR) light is electromagnetic radiation with longer wavelengths than those of visible light, extending from the nominal red edge of the visible spectrum at 0.74 micrometers (µm) to 1 mm. This range of wavelengths corresponds to a frequency range of approximately 300 GHz to 400 THz, and includes most of the thermal radiation emitted by objects near room temperature. Infrared light is emitted or absorbed by molecules when they change their rotational-vibrational movements.
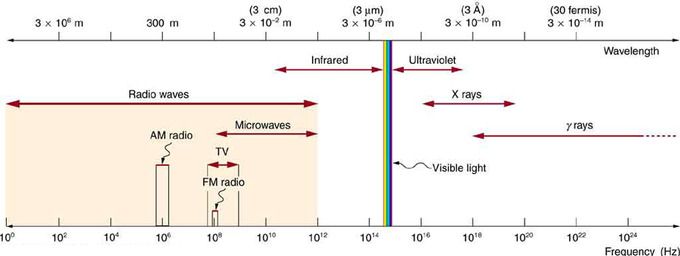
Electromagnetic Spectrum: The electromagnetic spectrum, showing the major categories of electromagnetic waves. The range of frequencies and wavelengths is remarkable. The dividing line between some categories is distinct, whereas other categories overlap. Microwaves encompass the high frequency portion of the radio section of the EM spectrum.
Subcategories of IR Waves
The infrared part of the electromagnetic spectrum covers the range from roughly 300 GHz (1 mm) to 400 THz (750 nm). It can be divided into three parts: It can be divided into three parts:
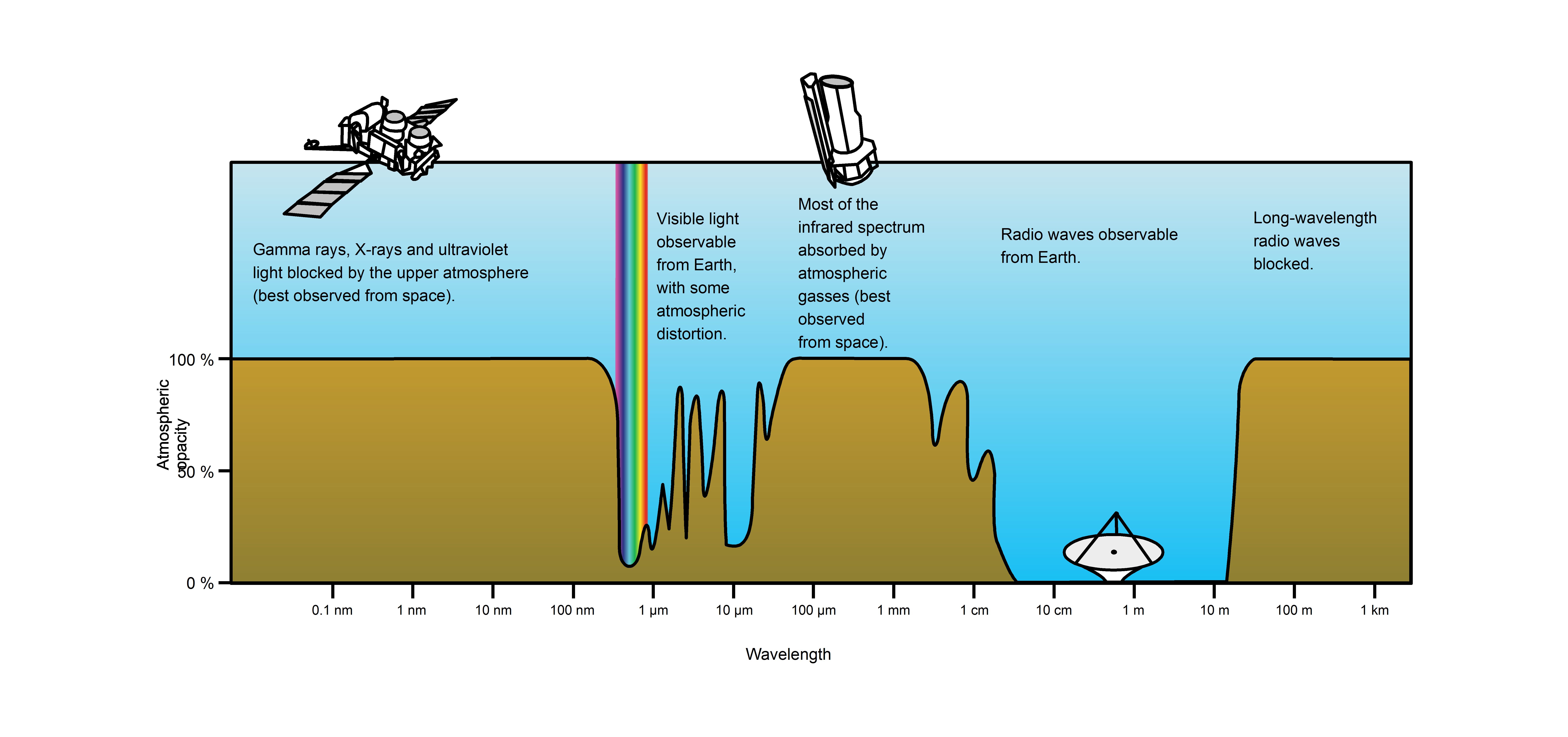
Atmospheric Transmittance: This is a plot of Earth’s atmospheric transmittance (or opacity) to various wavelengths of electromagnetic radiation. Most UV wavelengths are absorbed by oxygen and ozone in Earth’s atmosphere. Observations of astronomical UV sources must be done from space.
- Far-infrared, from 300 GHz (1 mm) to 30 THz (10 μm) – The lower part of this range may also be called microwaves. This radiation is typically absorbed by so-called rotational modes in gas-phase molecules, by molecular motions in liquids, and by phonons in solids. The water in Earth’s atmosphere absorbs so strongly in this range that it renders the atmosphere in effect opaque. However, there are certain wavelength ranges (“windows”) within the opaque range that allow partial transmission, and can be used for astronomy. The wavelength range from approximately 200 μm up to a few mm is often referred to as “sub-millimeter” in astronomy, reserving far infrared for wavelengths below 200 μm.
- Mid-infrared, from 30 to 120 THz (10 to 2.5 μm) – Hot objects (black-body radiators) can radiate strongly in this range, and human skin at normal body temperature radiates strongly at the lower end of this region. This radiation is absorbed by molecular vibrations, where the different atoms in a molecule vibrate around their equilibrium positions. This range is sometimes called the fingerprint region, since the mid-infrared absorption spectrum of a compound is very specific for that compound.
- Near-infrared, from 120 to 400 THz (2,500 to 750 nm) – Physical processes that are relevant for this range are similar to those for visible light. The highest frequences in this region can be detected directly by some types of photographic film, and by many types of solid state image sensors for infrared photography and videography.
Note that in some fields the boundaries of these categories differ slightly; for example, in astronomy “near-infrared” is considered to extend to 5 μm rather than 2.5 μm.
Heat and Thermal Radiation
Infrared radiation is popularly known as “heat radiation,” but light and electromagnetic waves of any frequency will heat surfaces that absorb them. Infrared light from the Sun only accounts for 49% of the heating of the Earth, with the rest being caused by visible light that is absorbed then re-radiated at longer wavelengths. Visible light or ultraviolet-emitting lasers can char paper and incandescently hot objects emit visible radiation. Objects at room temperature will emit radiation mostly concentrated in the 8 to 25 µm band, but this is not distinct from the emission of visible light by incandescent objects and ultraviolet by even hotter objects (see sections on black body radiation and Wien’s displacement law).
Heat is energy in transient form that flows due to temperature difference. Unlike heat transmitted by thermal conduction or thermal convection, radiation can propagate through a vacuum.
The concept of emissivity is important in understanding the infrared emissions of objects. This is a property of a surface which describes how its thermal emissions deviate from the ideal of a black body. To further explain, two objects at the same physical temperature will not “appear” the same temperature in an infrared image if they have differing emissivities.
Sources of IR Waves
As stated above, while infrared radiation is commonly referred to as heat radiation, only objects emitting with a certain range of temperatures and emissivities will produce most of their electromagnetic emission in the infrared part of the spectrum. However, this is the case for most objects and environments humans encounter in our daily lives. Humans, their surroundings, and the Earth itself emit most of their thermal radiation at wavelengths near 10 microns, the boundary between mid and far infrared according to the delineation above. The range of wavelengths most relevant to thermally emitting objects on earth is often called the thermal infrared. Many astronomical objects emit detectable amounts of IR radiation at non-thermal wavelengths.
Infrared radiation can be used to remotely determine the temperature of objects (if the emissivity is known). This is termed thermography, mainly used in military and industrial applications but the technology is reaching the public market in the form of infrared cameras on cars due to the massively reduced production costs.
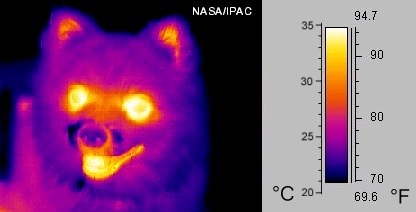
Thermography: A thermographic image of a dog
Applications of IR waves extend to heating, communication, meteorology, spectroscopy, astronomy, biological and medical science, and even the analysis of works of art.
Visible Light
Visible light is the portion of the electromagnetic spectrum that is visible to the human eye, ranging from roughly 390 to 750 nm.
Skills to Develop
- Distinguish six ranges of the visible spectrum
Visible Light
Visible light, as called the visible spectrum, is the portion of the electromagnetic spectrum that is visible to (can be detected by) the human eye. Electromagnetic radiation in this range of wavelengths is often simply referred to as “light”. A typical human eye will respond to wavelengths from about 390 to 750 nm (0.39 to 0.75 µm). In terms of frequency, this corresponds to a band in the vicinity of 400–790 THz. A light-adapted eye generally has its maximum sensitivity at around 555 nm (540 THz), in the green region of the optical spectrum. The spectrum does not, however, contain all the colors that the human eyes and brain can distinguish. Unsaturated colors such as pink, or purple variations such as magenta, are absent, for example, because they can be made only by a mix of multiple wavelengths.
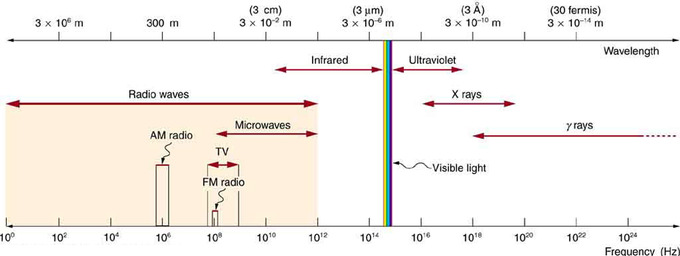
Electromagnetic Spectrum: The electromagnetic spectrum, showing the major categories of electromagnetic waves. The range of frequencies and wavelengths is remarkable. The dividing line between some categories is distinct, whereas other categories overlap. Microwaves encompass the high frequency portion of the radio section of the EM spectrum.
Visible light is produced by vibrations and rotations of atoms and molecules, as well as by electronic transitions within atoms and molecules. The receivers or detectors of light largely utilize electronic transitions. We say the atoms and molecules are excited when they absorb and relax when they emit through electronic transitions.
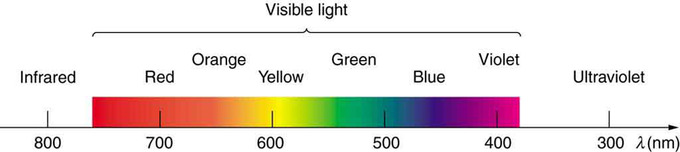
Visible Spectrum: A small part of the electromagnetic spectrum that includes its visible components. The divisions between infrared, visible, and ultraviolet are not perfectly distinct, nor are those between the seven rainbow colors.
The figure above shows this part of the spectrum, together with the colors associated with particular pure wavelengths. Red light has the lowest frequencies and longest wavelengths, while violet has the highest frequencies and shortest wavelengths. Blackbody radiation from the Sun peaks in the visible part of the spectrum but is more intense in the red than in the violet, making the Sun yellowish in appearance.
Colors that can be produced by visible light of a narrow band of wavelengths (monochromaticlight) are called pure spectral colors. Quantitatively, the regions of the visible spectrum encompassing each spectral color can be delineated roughly as:
- red – 620 to 750 nm (400-484 THz)
Note that each color can come in many shades, since the spectrum is continuous. The human eye is insensitive to electromagnetic radiation outside this range. By definition any images presented with data recorded from wavelengths other than those in the visible part of the spectrum (such as IR images of humans or animals or astronomical X-ray images) are necessarily in false color.
Visible Light and Earth’s Atmosphere
Visible wavelengths pass through the “optical window”, the region of the electromagnetic spectrum which allows wavelengths to pass largely unattenuated through the Earth’s atmosphere (see opacity plot in. An example of this phenomenon is that clean air scatters blue light more than red wavelengths, and so the midday sky appears blue.
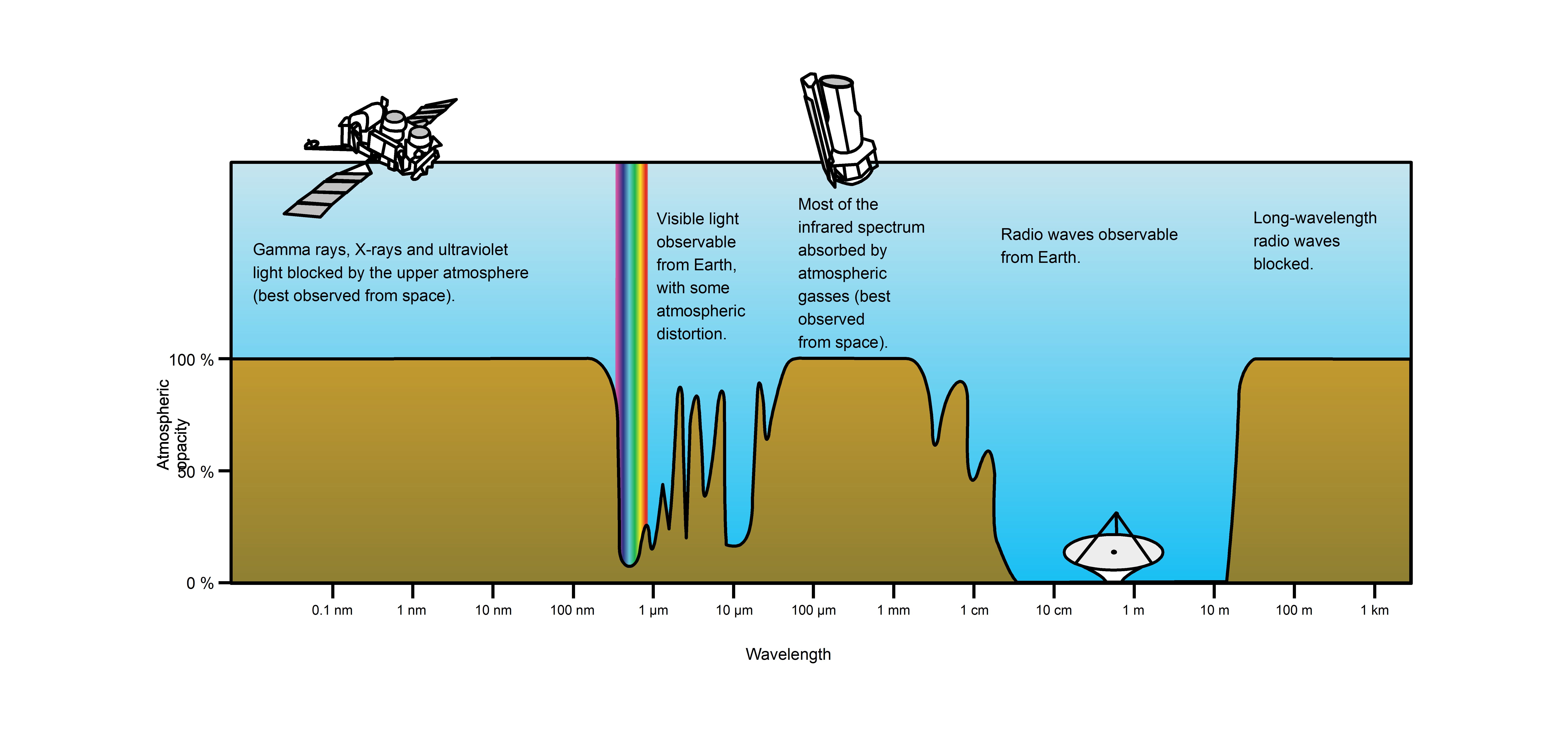
Atmospheric Transmittance: This is a plot of Earth’s atmospheric transmittance (or opacity) to various wavelengths of electromagnetic radiation. Most UV wavelengths are absorbed by oxygen and ozone in Earth’s atmosphere. Observations of astronomical UV sources must be done from space.
The optical window is also called the visible window because it overlaps the human visible response spectrum. This is not coincidental as humanity’s ancestors evolved vision that could make use of the most plentiful wavelengths of light.The near infrared (NIR) window lies just out of the human vision, as well as the Medium Wavelength IR (MWIR) window and the Long Wavelength or Far Infrared (LWIR or FIR) window though other animals may experience them.
A consequence of the existence of the optical window in Earth’s atmosphere is the relatively balmy temperature conditions on Earth’s surface. The Sun’s luminosity function peaks in the visible range and light in that range is able to travel to the surface of the planet unattenuated due to the optical window. This allows visible light to heat the surface. The surface of the planet then emits energy primarily in infrared wavelengths, which has much greater difficulty escaping (and thus causing the planet to cool) due to the opacity of the atmosphere in the infrared. Earth’s surface would be much cooler without this effect.
Photosynthesis
Plants, like animals, have evolved to utilize and respond to parts of the electromagnetic spectrum they are embedded in. Plants (and many bacteria) convert the light energy captured from the Sun into chemical energy that can be used to fuel the organism’s activities. In plants, algae, and cyanobacteria, photosynthesis uses carbon dioxide and water, releasing oxygen as a waste product. Photosynthesis is vital for all aerobic life on Earth (such as humans and animals). The portion of the EM spectrum used by photosynthesic organisms is called the photosynthetically active region (PAR) and corresponds to solar radiation between 400 and 700 nm, substantially overlapping with the range of human vision. This is again not coincidental; the light in this range is the most plentiful to organisms on the surface of Earth because the Sun emits about half of its luminosity in this wavelength range and it is allowed to pass freely through the optical windows in Earth’s atmosphere.
Ultraviolet Light
Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light in the range 10 nm to 400 nm.
Skills to Develop
- Identify wavelength range characteristic for ultraviolet light and its biological effects
Ultraviolet Light
Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, that is, in the range 10 nm to 400 nm, corresponding to photon energies from 3 eV to 124 eV (1 eV = 1.6e-19 J; EM radiation with frequencies higher than those of visible light are often expressed in terms of energy rather than frequency). It is so-named because the spectrum consists of electromagnetic waves with frequencies higher than those that humans identify as the color violet. These frequencies are invisible to humans, but visible to a number of insects and birds.
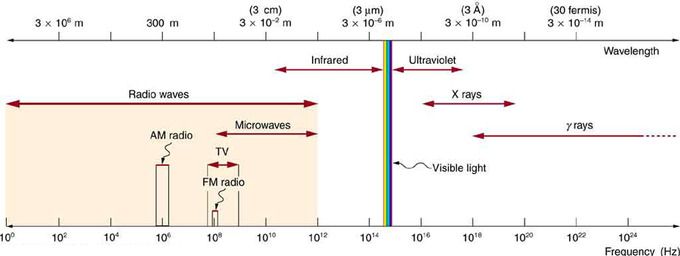
Electromagnetic Spectrum: The electromagnetic spectrum, showing the major categories of electromagnetic waves. The range of frequencies and wavelengths is remarkable. The dividing line between some categories is distinct, whereas other categories overlap. Microwaves encompass the high frequency portion of the radio section of the EM spectrum.
UV light is found in sunlight (where it constitutes about 10% of the energy in vacuum) and is emitted by electric arcs and specialized lights such as black lights. It can cause chemical reactions, and causes many substances to glow or fluoresce. Most ultraviolet is classified as non-ionizing radiation. The higher energies of the ultraviolet spectrum from wavelengths about 10 nm to 120 nm (‘extreme’ ultraviolet) are ionizing, but this type of ultraviolet in sunlight is blocked by normal molecular oxygen (O2) in air, and does not reach the ground. However, the entire spectrum of ultraviolet radiation has some of the biological features of ionizing radiation, in doing far more damage to many molecules in biological systems than is accounted for by simple heating effects (an example is sunburn). These properties derive from the ultraviolet photon’s power to alter chemical bonds in molecules, even without having enough energy to ionize atoms.
Although ultraviolet radiation is invisible to the human eye, most people are aware of the effects of UV on the skin, called suntan and sunburn. In addition to short wave UV blocked by oxygen, a great deal (>97%) of mid-range ultraviolet (almost all UV above 280 nm and most up to 315 nm) is blocked by the ozone layer, and like ionizing short wave UV, would cause much damage to living organisms if it penetrated the atmosphere. After atmospheric filtering, only about 3% of the total energy of sunlight at the zenith is ultraviolet, and this fraction decreases at other sun angles. Much of it is near-ultraviolet that does not cause sunburn, but is still capable of causing long term skin damage and cancer. An even smaller fraction of ultraviolet that reaches the ground is responsible for sunburn and also the formation of vitamin D (peak production occurring between 295 and 297 nm) in all organisms that make this vitamin (including humans). The UV spectrum thus has many effects, both beneficial and damaging, to human health.
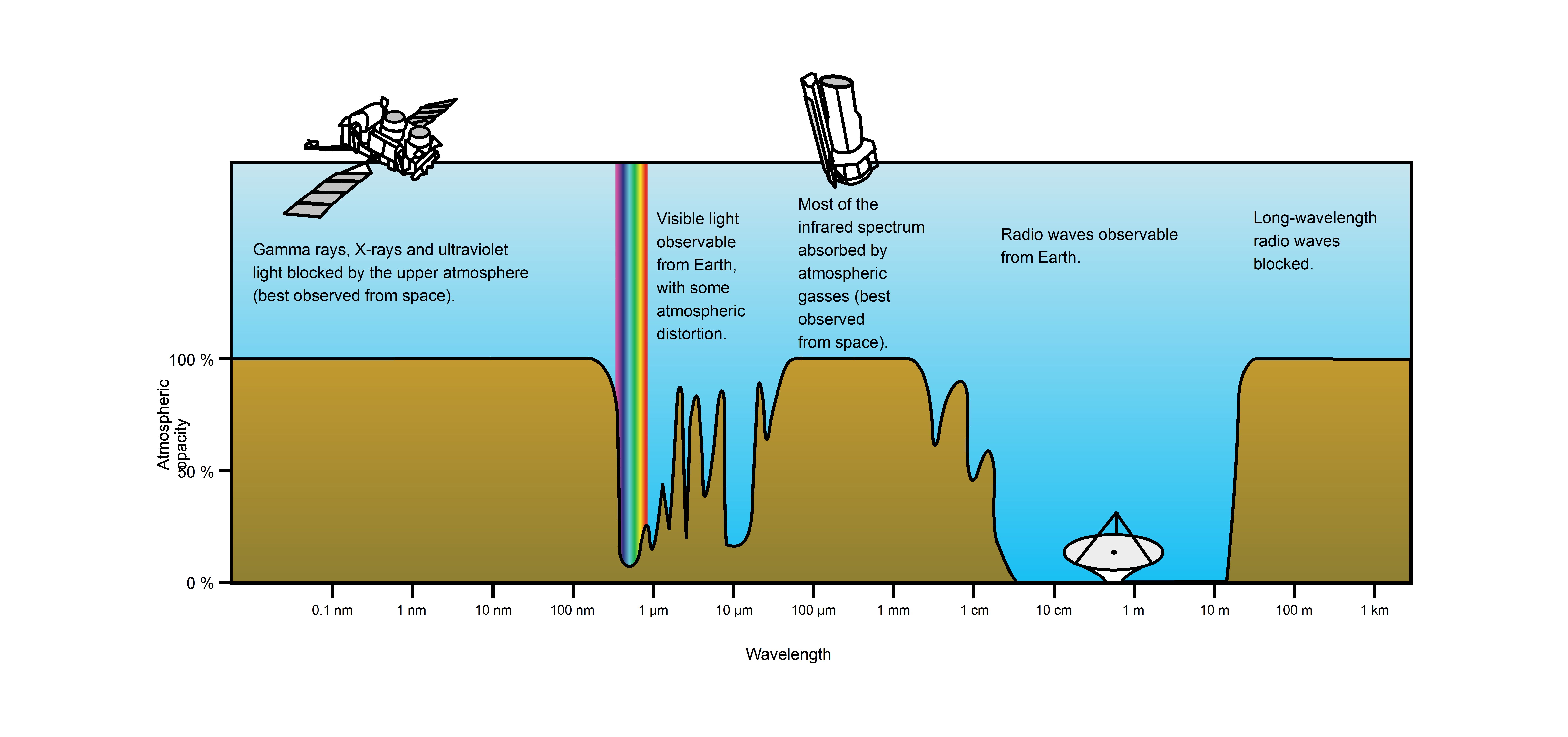
Atmospheric Transmittance: This is a plot of Earth’s atmospheric opacity (opposite of transmittance) to various wavelengths of electromagnetic radiation, including visible light. Visible light passes relatively unimpeded through the atmosphere in the “optical window.” Most UV wavelengths are absorbed by oxygen and ozone in Earth’s atmosphere. Observations of astronomical UV sources must be done from space.
Subcategories of UV Light
Solar UV radiation is commonly subdivided into three regions: UV-A (320–400 nm), UV-B (290–320 nm), and UV-C (220–290 nm), ranked from long to shorter wavelengths (from smaller to larger energies). Most UV-B and all UV-C is absorbed by ozone (O3) molecules in the upper atmosphere. Consequently, 99% of the solar UV radiation reaching the Earth’s surface is UV-A.
There are other schemes for dividing UV into different categories, another common one is: near-ultraviolet (NUV – 300-400 nm), middle ultraviolet (MUV – 200-300 nm), far ultraviolet (FUV – 200-122 nm), and extreme ultraviolet (EUV- 121-10 nm).
Harmful Effects
An overexposure to UVB radiation can cause sunburn and some forms of skin cancer. In humans, prolonged exposure to solar UV radiation may result in acute and chronic health effects on the skin, eye, and immune system. Moreover, UVC can cause adverse effects that can variously be mutagenic or carcinogenic.
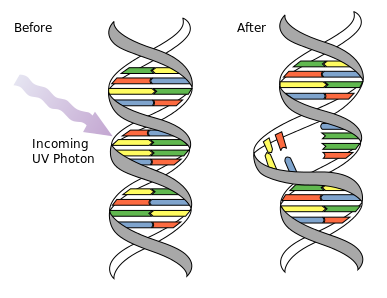
DNA UV Mutation: Ultraviolet photons harm the DNA molecules of living organisms in different ways. In one common damage event, adjacent thymine bases bond with each other, instead of across the “ladder. ” This “thymine dimer” makes a bulge, and the distorted DNA molecule does not function properly.
The International Agency for Research on Cancer of the World Health Organization has classified all categories and wavelengths of ultraviolet radiation as a Group 1 carcinogen. This is the highest level designation for carcinogens and means that “there is enough evidence to conclude that it can cause cancer in humans. ”
Beneficial Effects
UVB exposure induces the production of vitamin D in the skin. The majority of positive health effects are related to this vitamin. It has regulatory roles in calcium metabolism (which is vital for normal functioning of the nervous system, as well as for bone growth and maintenance of bone density), immunity, cell proliferation, insulin secretion, and blood pressure.
X-Rays
X-rays are electromagnetic waves with wavelengths in the range of 0.01 to 10 nanometers and energies in the range of 100 eV to 100 keV.
Skills to Develop
- Distinguish two categories of X-rays and their biological effects
X-Rays
X-rays are electromagnetic waves with wavelengths in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz (3×1016 Hz to 3×1019 Hz) and energies in the range 100 eV to 100 keV. They are shorter in wavelength than UV rays and longer than gamma rays. In many languages, X-radiation is called Röntgen radiation, after Wilhelm Röntgen, who is usually credited as its discoverer, and who had named it X-radiation to signify an unknown type of radiation.

Electromagnetic Spectrum: The electromagnetic spectrum, showing the major categories of electromagnetic waves. The range of frequencies and wavelengths is remarkable. The dividing line between some categories is distinct, whereas other categories overlap. Microwaves encompass the high frequency portion of the radio section of the EM spectrum.
Properties and Applications
X-ray photons carry enough energy to ionize atoms and disrupt molecular bonds. This makes it a type of ionizing radiation and thereby harmful to living tissue. A very high radiation dose over a short amount of time causes radiation sickness, while lower doses can give an increased risk of radiation-induced cancer. In medical imaging this increased cancer risk is generally greatly outweighed by the benefits of the examination. The ionizing capability of X-rays can be utilized in cancer treatment to kill malignant cells using radiation therapy. It is also used for material characterization using X-ray spectroscopy.
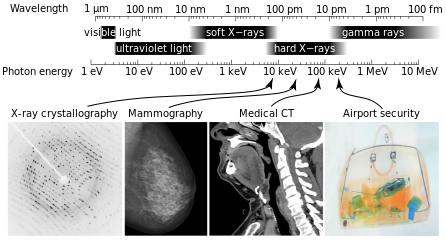
X-Ray Spectrum and Applications: X-rays are part of the electromagnetic spectrum, with wavelengths shorter than those of visible light. Different applications use different parts of the X-ray spectrum.
X-rays with photon energies above 5 to 10 keV (below 0.2-0.1 nm wavelength), are called hard X-rays, while those with lower energy are called soft X-rays. Due to their penetrating ability, hard X-rays are widely used to image the inside of objects (e.g., in medical radiography and airport security). As a result, the term X-ray is metonymically used to refer to a radiographic image produced using this method, in addition to the method itself. Since the wavelength of hard X-rays are similar to the size of atoms, they are also useful for determining crystal structures by X-ray crystallography. By contrast, soft X-rays are easily absorbed in air and the attenuation length of 600 eV (~2 nm) X-rays in water is less than 1 micrometer.
In medical diagnostic applications, the low energy (soft) X-rays are unwanted, since they are totally absorbed by the body, increasing the radiation dose without contributing to the image. Hence, a thin metal sheet, often of aluminum, called an X-ray filter, is usually placed over the window of the X-ray tube, absorbing the low energy part in the spectrum. This is called hardening the beam since it shifts the center of the spectrum towards higher energy (or harder) X-rays.
Distinction Between X-Rays and Gamma Rays
The distinction between X-rays and gamma rays is somewhat arbitrary. The most frequent method of distinguishing between X- and gamma radiation is the basis of wavelength, with radiation shorter than some arbitrary wavelength, such as 10−11 m, defined as gamma rays. The electromagnetic radiation emitted by X-ray tubes generally has a longer wavelength than the radiation emitted by radioactive nuclei. Historically, therefore, an alternative means of distinguishing between the two types of radiation has been by their origin: X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus. There is overlap between the wavelength bands of photons emitted by electrons outside the nucleus, and photons emitted by the nucleus. Like all electromagnetic radiation, the properties of X-rays (or gamma rays) depend only on their wavelength and polarization.
Gamma Rays
Gamma rays are very high frequency electromagnetic waves usually emitted from radioactive decay with frequencies greater than 1019Hz.
Skills to Develop
- Identify wavelength range characteristic for gamma rays, noting their biological effects and distinguishing them from gamma rays
Gamma Rays
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency and therefore high energy. Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (less than the diameter of an atom). However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes. Gamma rays from radioactive decay are defined as gamma rays no matter what their energy, so that there is no lower limit to gamma energy derived from radioactive decay. Gamma decay commonly produces energies of a few hundred keV, and almost always less than 10 MeV.
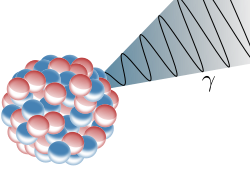
Gamma Decay: Illustration of an emission of a gamma ray (γ) from an atomic nucleus
Gamma rays are ionizing radiation and are thus biologically hazardous. They are classically produced by the decay from high energy states of atomic nuclei, a process called gamma decay, but are also created by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium during its gamma decay. Villard’s radiation was named “gamma rays” by Ernest Rutherford in 1903.
Gamma Ray Sources
Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes such as potassium-40, and also as a secondary radiation from various atmospheric interactions with cosmic ray particles. Some rare terrestrial natural sources that produce gamma rays that are not of a nuclear origin, are lightning strikes and terrestrial gamma-ray flashes, which produce high energy emissions from natural high-energy voltages. Gamma rays are produced by a number of astronomical processes in which very high-energy electrons are produced. Such electrons produce secondary gamma rays by the mechanisms of bremsstrahlung, inverse Compton scattering and synchrotron radiation. A large fraction of such astronomical gamma rays are screened by Earth’s atmosphere and must be detected by spacecraft. Notable artificial sources of gamma rays include fission such as occurs in nuclear reactors, and high energy physics experiments, such as neutral pion decay and nuclear fusion.
Gamma Rays vs. X-Rays
Gamma rays have characteristics identical to X-rays of the same frequency—they differ only in source. At higher frequencies, γ rays are more penetrating and more damaging to living tissue. They have many of the same uses as X-rays, including cancer therapy. Gamma radiation from radioactive materials is used in nuclear medicine.
The distinction between X-rays and gamma rays has changed in recent decades. Originally, the electromagnetic radiation emitted by X-ray tubes almost invariably had a longer wavelength than the radiation (gamma rays) emitted by radioactive nuclei. Older literature distinguished between X- and gamma radiation on the basis of wavelength, with radiation shorter than some arbitrary wavelength, such as 10−11 m, defined as gamma rays. However, with artificial sources now able to duplicate any electromagnetic radiation that originates in the nucleus, as well as far higher energies, the wavelengths characteristic of radioactive gamma ray sources vs. other types, now completely overlap. Thus, gamma rays are now usually distinguished by their origin: X-rays are emitted by definition by electrons outside the nucleus, while gamma rays are emitted by the nucleus.
Exceptions to this convention occur in astronomy, where gamma decay is seen in the afterglow of certain supernovas, but other high energy processes known to involve other than radioactive decay are still classed as sources of gamma radiation. A notable example is extremely powerful bursts of high-energy radiation normally referred to as long duration gamma-ray bursts, which produce gamma rays by a mechanism not compatible with radioactive decay. These bursts of gamma rays, thought to be due to the collapse of stars called hypernovas, are the most powerful events so far discovered in the cosmos. Astrophysical processes are the only sources for very high energy gamma rays (~100 MeV).
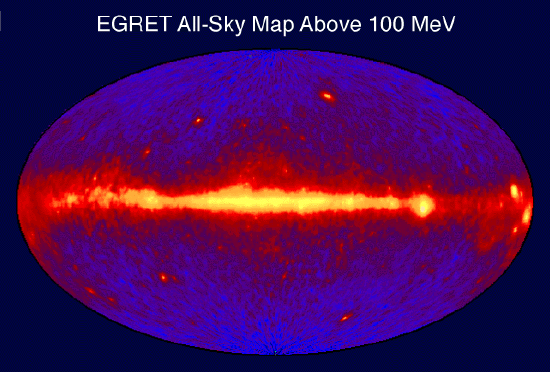
Gamma Ray Sky Map: This is an image of the entire sky in 100 MeV or greater gamma rays as seen by the EGRET instrument aboard the CGRO spacecraft. Bright spots within the galactic plane are pulsars (spinning neutron stars with strong magnetic fields), while those above and below the plane are thought to be quasars (galaxies with supermassive black holes actively accreting matter).
Health Effects
All ionizing radiation causes similar damage at a cellular level, but because rays of alpha particles and beta particles are relatively non-penetrating, external exposure to them causes only localized damage (e.g., radiation burns to the skin). Gamma rays and neutrons are more penetrating, causing diffuse damage throughout the body (e.g., radiation sickness, cell’s DNA damage, cell death due to damaged DNA, increasing incidence of cancer) rather than burns. External radiation exposure should also be distinguished from internal exposure, due to ingested or inhaled radioactive substances, which, depending on the substance’s chemical nature, can produce both diffuse and localized internal damage. The most biological damaging forms of gamma radiation occur at energies between 3 and 10 MeV.
Key Points
- The lowest frequency portion of the electromagnetic spectrum is designated as “radio,” generally considered to have wavelengths within 1 millimeter to 100 kilometers or frequencies within 300 GHz to 3 kHz.
- There is a wide range of subcategories contained within radio including AM and FM radio. Radio waves can be generated by natural sources such as lightning or astronomical phenomena; or by artificial sources such as broadcast radio towers, cell phones, satellites and radar.
- AM radio waves are used to carry commercial radio signals in the frequency range from 540 to 1600 kHz. The abbreviation AM stands for amplitude modulation—the method for placing information on these waves. AM waves have constant frequency, but a varying amplitude.
- FM radio waves are also used for commercial radio transmission in the frequency range of 88 to 108 MHz. FM stands for frequency modulation, which produces a wave of constant amplitude but varying frequency.
- The microwave region of the electromagnetic (EM) spectrum is generally considered to overlap with the highest frequency (shortest wavelength ) radio waves.
- The prefix “micro-” in “microwave” is not meant to suggest a wavelength in the micrometer range. It indicates that microwaves are “small” compared to waves used in typical radio broadcasting in that they have shorter wavelengths.
- The microwave portion of the electromagnetic spectrum can be subdivided into three ranges listed below from high to low frequencies: extremely high frequency (30 to 300 GHz), super high frequency (3 to 30 GHz), and ultra-high frequency (300 MHz to 3 GHz).
- Microwave sources include artificial devices such as circuits, transmission towers, radar, masers, and microwave ovens, as well as natural sources such as the Sun and the Cosmic Microwave Background.
- Microwaves can also be produced by atoms and molecules. They are, for example, a component of electromagnetic radiation generated by thermal agitation. The thermal motion of atoms and molecules in any object at a temperature above absolute zero causes them to emit and absorb radiation.
- Infrared light includes most of the thermal radiation emitted by objects near room temperature. Infrared light is emitted or absorbed by molecules when they change their rotational-vibrational movements.
- The infrared portion of the spectrum can be divided into three regions in wavelength: far-infrared, from 300 GHz (1 mm) to 30 THz (10 μm); mid-infrared, from 30 to 120 THz (10 to 2.5 μm); and near-infrared, from 120 to 400 THz (2,500 to 750 nm).
- Infrared radiation is popularly known as ” heat radiation,” but light and electromagnetic waves of any frequency will heat surfaces that absorb them.
- The concept of emissivity is important in understanding the infrared emissions of objects. This is a property of a surface which describes how its thermal emissions deviate from the ideal of a black body.
- Infrared radiation can be used to remotely determine the temperature of objects (if the emissivity is known). This is termed thermography, mainly used in military and industrial applications.
- Visible light is produced by vibrations and rotations of atoms and molecules, as well as by electronic transitions within atoms and molecules. We say the atoms and molecules are excited when they absorb and relax when they emit through electronic transitions.
- This figure shows the visible part of the spectrum, together with the colors associated with particular pure wavelengths. Red light has the lowest frequencies and longest wavelengths, while violet has the highest frequencies and shortest wavelengths.
- Colors that can be produced by visible light of a narrow band of wavelengths are called pure spectral colors. They can be delineated roughly in wavelength as: violet (380-450 nm), blue (450-495 nm), green (495-570 nm), yellow (570-590 nm), orange (590-620 nm), and red (620 to 750 nm).
- Visible wavelengths pass through the optical window, the Earth’s atmosphere allows this region of the electromagnetic spectrum to pass through largely unattenuated (see opacity plot in.
- The portion of the EM spectrum used by photosynthesic organisms is called the photosynthetically active region (PAR) and corresponds to solar radiation between 400 and 700 nm, substantially overlapping with the range of human vision.
- Ultraviolet light gets its name because the spectrum consists of electromagnetic waves with frequencies higher than those that humans identify as the color violet.
- Most UV is non- ionizing radiation, though UV with higher energies (10-120 nm) is ionizing. All UV can have harmful effects on biological matter (such as causing cancers) with the highest energies causing the most damage.
- The danger posed by lower energy UV radiation is derived from the ultraviolet photon ‘s power to alter chemical bonds in molecules, even without having enough energy to ionize atoms.
- Solar UV radiation is commonly subdivided into three regions: UV-A (320–400 nm), UV-B (290–320 nm), and UV-C (220–290 nm), ranked from long to shorter wavelengths (from smaller to larger energies).
- Most UV-B and all UV-C is absorbed by ozone (O3) molecules in the upper atmosphere. Consequently, 99% of the solar UV radiation reaching the Earth’s surface is UV-A.
- X-rays have shorter wavelengths (higher energy ) than UV waves and, generally, longer wavelengths (lower energy) than gamma rays. Sometimes X-rays are called Röntgen radiation, after Wilhelm Röntgen, who is usually credited as their discoverer.
- Because X-rays have very high energy they are known as ionizing radiation and can harm living tissue. A very high radiation dose over a short amount of time causes radiation sickness, while lower doses can give an increased risk of radiation-induced cancer.
- Lower doses of X-ray radiation can be very effectively used in medical radiography and X-ray spectroscopy. In the case of medical radiography, the benefits of using X-rays for examination far outweighs the risk.
- X-rays are broken up into broad two categories: hard X-rays with energies above 5-10 keV (below 0.2-0.1 nm wavelength) and soft X-rays with energies 100 eV – 5 keV (10 – 0.1 nm wavelength). Hard X-rays are more useful for radiography because they pass through tissue.
- The distinction between X-rays and gamma rays is somewhat arbitrary and there is substantial overlap at the high energy boundary. However, in general they are distinguished by their source, with gamma rays originating from the nucleus and X-rays from the electrons in the atom.
- Gamma rays are the highest energy EM radiation and typically have energies greater than 100 keV, frequencies greater than 1019 Hz, and wavelengths less than 10 picometers.
- Gamma rays from radioactive decay are defined as gamma rays no matter what their energy, so that there is no lower limit to gamma energy derived from radioactive decay. Gamma decay commonly produces energies of a few hundred keV, and almost always less than 10 MeV.
- Gamma rays have characteristics identical to X-rays of the same frequency—they differ only in source. Gamma rays are usually distinguished by their origin: X-rays are emitted by definition by electrons outside the nucleus, while gamma rays are emitted by the nucleus.
- Natural sources of gamma rays include gamma decay from naturally occurring radioisotopes such as potassium-40, and also as a secondary radiation from atmospheric interactions with cosmic ray particles. Exotic astrophysical processes will also produce gamma rays.
- Gamma rays are ionizing radiation and are thus biologically hazardous. The most biological damaging forms of gamma radiation occur at energies between 3 and 10 MeV.
Key Terms
- AM radio waves: Waves used to carry commercial radio signals between 540 and 1600 kHz. Information is carried by amplitude variation, while the frequency remains constant.
- FM radio waves: Waves used to carry commercial radio signals between 88 and 108 MHz. Information is carried by frequency modulation, while the signal amplitude remains constant.
- radio waves: Designates a portion of the electromagnetic spectrum having frequencies ranging from 300 GHz to 3 kHz, or equivalently, wavelengths from 1 millimeter to 100 kilometers.
- terahertz radiation: Electromagnetic waves with frequencies around one terahertz.
- thermal agitation: The thermal motion of atoms and molecules in any object at a temperature above absolute zero, causing them to emit and absorb radiation.
- radar: A method of detecting distant objects and determining their position, velocity, or other characteristics by analysis of sent radio waves (usually microwaves) reflected from their surfaces.
- emissivity: The energy-emitting propensity of a surface, usually measured at a specific wavelength.
- thermography: Any of several techniques for the remote measurement of the temperature variations of a body, especially by creating images produced by infrared radiation.
- thermal radiation: The electromagnetic radiation emitted from a body as a consequence of its temperature; increasing the temperature of the body increases the amount of radiation produced, and shifts it to shorter wavelengths (higher frequencies) in a manner explained only by quantum mechanics.
- spectral color: a color that is evoked by a single wavelength of light in the visible spectrum, or by a relatively narrow band of wavelengths. Every wavelength of light is perceived as a spectral color, in a continuous spectrum; the colors of sufficiently close wavelengths are indistinguishable.
- optical window: the optical portion of the electromagnetic spectrum that passes through the atmosphere all the way to the ground. The window runs from around 300 nanometers (ultraviolet-C) at the short end up into the range the eye can use, roughly 400-700 nm and continues up through the visual infrared to around 1100 nm, which is thermal infrared.
- visible light: the part of the electromagnetic spectrum, between infrared and ultraviolet, that is visible to the human eye
- ozone layer: A region of the stratosphere, between 15 and 30 kilometres in altitude, containing a relatively high concentration of ozone; it absorbs most solar ultraviolet radiation.
- ionizing radiation: high-energy radiation that is capable of causing ionization in substances through which it passes; also includes high-energy particles
- non-ionizing radiation: Radiation that does not cause atmospheric ionization; electrically neutral radiation.
- X-ray spectroscopy: The use of an X-ray spectrometer for chemical analysis.
- x-ray crystallography: A technique in which the patterns formed by the diffraction of X-rays on passing through a crystalline substance yield information on the lattice structure of the crystal, and the molecular structure of the substance.
- radiograph: An image, often a photographic negative, produced by radiation other than normal light; especially an X-ray photograph.
- gamma ray: A very high frequency (and therefore very high energy) electromagnetic radiation emitted as a consequence of radioactivity.
- gamma decay: A nuclear reaction with the emission of a gamma ray.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax College, College Physics. September 18, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Radio waves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radio_waves. License: CC BY-SA: Attribution-ShareAlike
- Radio spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radio_spectrum. License: CC BY-SA: Attribution-ShareAlike
- Radio frequency. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radio_frequency. License: CC BY-SA: Attribution-ShareAlike
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/definition/radio-waves. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/definition/fm-radio-waves. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/definition/am-radio-waves. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- terahertz radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/terahertz_radiation. License: CC BY-SA: Attribution-ShareAlike
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum. License: CC BY-SA: Attribution-ShareAlike
- Extremely high frequency. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Extremely_high_frequency. License: CC BY-SA: Attribution-ShareAlike
- Ultra high frequency. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Ultra_high_frequency. License: CC BY-SA: Attribution-ShareAlike
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: CC BY-SA: Attribution-ShareAlike
- Super high frequency. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Super_high_frequency. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. September 18, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/definition/thermal-agitation. License: CC BY-SA: Attribution-ShareAlike
- radar. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radar. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. September 18, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Infrared radiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Infrared_radiation. License: CC BY-SA: Attribution-ShareAlike
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum. License: CC BY-SA: Attribution-ShareAlike
- emissivity. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/emissivity. License: CC BY-SA: Attribution-ShareAlike
- thermography. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/thermography. License: CC BY-SA: Attribution-ShareAlike
- thermal radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/thermal_radiation. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- Infrared radiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Infrared_radiation. License: Public Domain: No Known Copyright
- Visible spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Visible_spectrum. License: CC BY-SA: Attribution-ShareAlike
- Spectral color. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Spectral_color. License: CC BY-SA: Attribution-ShareAlike
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. September 18, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- spectral color. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/spectral%20color. License: CC BY-SA: Attribution-ShareAlike
- optical window. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/optical%20window. License: CC BY-SA: Attribution-ShareAlike
- visible light. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/visible_light. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- Infrared radiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Infrared_radiation. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 19, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- ozone layer. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ozone_layer. License: CC BY-SA: Attribution-ShareAlike
- ionizing radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ionizing_radiation. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. September 18, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum. License: CC BY-SA: Attribution-ShareAlike
- non-ionizing radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/non-ionizing_radiation. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- Infrared radiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Infrared_radiation. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 19, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Ultraviolet. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Ultraviolet. License: Public Domain: No Known Copyright
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- X-rays. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/X-rays. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. September 18, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum. License: CC BY-SA: Attribution-ShareAlike
- radiograph. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radiograph. License: CC BY-SA: Attribution-ShareAlike
- x-ray crystallography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/x-ray%20crystallography. License: CC BY-SA: Attribution-ShareAlike
- X-ray spectroscopy. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/X-ray_spectroscopy. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- Infrared radiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Infrared_radiation. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 19, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Ultraviolet. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Ultraviolet. License: Public Domain: No Known Copyright
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- X-rays. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/X-rays. License: Public Domain: No Known Copyright
- ionizing radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ionizing_radiation. License: CC BY-SA: Attribution-ShareAlike
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum. License: CC BY-SA: Attribution-ShareAlike
- gamma ray. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/gamma_ray. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. September 18, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Gamma rays. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Gamma_rays. License: CC BY-SA: Attribution-ShareAlike
- gamma decay. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/gamma_decay. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 17, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Microwaves. Provided by: Wikipedia. Located at: http://en.Wikipedia.org/wiki/Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- Infrared radiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Infrared_radiation. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. December 19, 2012. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Ultraviolet. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Ultraviolet. License: Public Domain: No Known Copyright
- Electromagnetic spectrum. Provided by: Wikipedia. Located at: http://en.Wikipedia.org/wiki/Electromagnetic_spectrum%23Microwaves. License: Public Domain: No Known Copyright
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. April 28, 2014. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42444/latest/?collection=col11406/1.7. License: CC BY: Attribution
- X-rays. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/X-rays. License: Public Domain: No Known Copyright
- Gamma rays. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Gamma_rays. License: Public Domain: No Known Copyright
- Gamma rays. Provided by: Wikipedia. Located at: http://en.Wikipedia.org/wiki/Gamma_rays. License: Public Domain: No Known Copyright

