4.2: Thermal Radiation
( \newcommand{\kernel}{\mathrm{null}\,}\)
4.2.1 Introduction to Thermal Radiation
All objects, regardless of temperature, have some internal motion of their molecules. The molecules in fluids such as liquids and gases freely move around and collide with one another. In solids, the molecules are held in place, but still vibrate. As a result, all objects emit some form of thermal radiation. Temperature also determines the average speed of gas molecules, which has a big impact on the composition of a planet’s atmosphere. Tiny Mercury’s weak gravity is not strong enough to retain any gases as they heat up from the Sun’s rays, same with the Moon. Earth, on the other hand, has a strong enough gravity to retain many gases, including oxygen, nitrogen, carbon dioxide and water vapor, but not lighter ones like hydrogen and helium. These gases were likely the main components of Earth’s atmosphere, but they quickly escaped into space. In contrast, Mars has a weaker gravity and has lost much of its early atmosphere, leaving only a thin layer of mostly the heavier gas, carbon dioxide. Even to this day, Mars is still losing its atmosphere to space.
At temperatures found on Earth, the thermal radiation emitted is in the infrared range of the spectrum and is, of course, invisible to the naked eye. This radiation can, however, be detected by infrared cameras. Firefighters can use such cameras to find unconscious victims inside a smoke-filled room where visible light is scattered by the smoke, but the infrared radiation is not.
 At typical Earth temperatures, all objects emit infrared radiation that can be detected with special cameras.
At typical Earth temperatures, all objects emit infrared radiation that can be detected with special cameras.
https:/www.flickr.com/photos/la1e/2689943235;
Recall in Chapter 3 that absolute zero on the Kelvin scale is the point where are motion ceases. An object that temperature would not emit any thermal radiation. In practice, however, we cannot reach absolute zero. We have been able to cool things to very close to absolute zero, even within a billionth of a degree, but it is not possible to get all the way down to absolute zero.
4.2.2 Blackbody Radiation
Physicists use a model called a blackbody, which a body whose radiation emitted is dependent only on its temperature. While a blackbody spectrum is an idealized model, many objects, including planets and stars, behave enough like a blackbody that it is a useful approximation of their spectra. An object’s thermal radiation spectrum depends on its temperature, with hotter objects emitting more light at all wavelengths per unit area and hotter objects emit photons with a higher average energy. The emission of thermal energy is governed by two key laws:
1. Peak wavelength is inversely proportional to temperature.
2. Total energy emitted is proportional to fourth power of temperature. E α T4
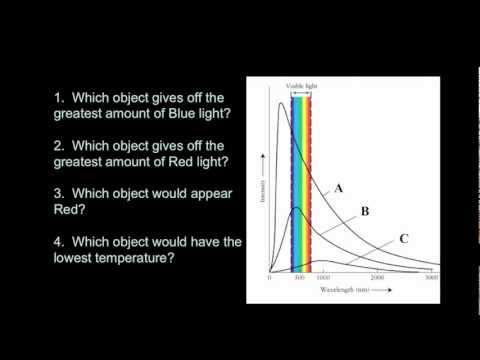
 Blackbody spectrum has a continuous spectrum with a peak wavelength based on its temperature.
Blackbody spectrum has a continuous spectrum with a peak wavelength based on its temperature.
Note that this is one of these instances where the relationship between energy and temperature in which energy most be given on the absolute (Kelvin) scale since negative temperature values would through these calculations off.
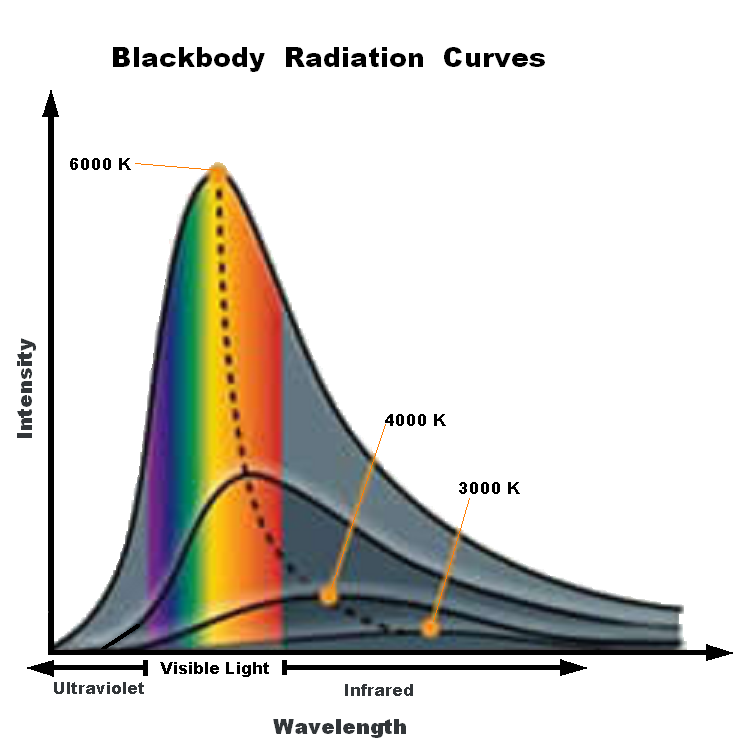
Wilhelm Wien studied the spectrum of objects as their temperatures increased. For example, a piece of metal at room temperature emits only infrared radiation and therefore, does not glow in visible light. If you heat it up, it will start to glow red, as the peak wavelength of the light emitted gets short. Heat it up even more, and it will glow blueish-white, showing that ite peak wavelength has shifter to even shorter (bluer) wavelengths. Wien’s Law describes how the peak wavelength of the radiation emitted by an object varies with temperature. All bodies emit thermal radiation spanning a broad range of wavelengths. The amount and peak wavelength of the radiation depends on the temperature of the body, but not on its composition. It is independent of what the material is made of. The higher the temperature, the more radiation is emitted and the shorter (or bluer) the peak wavelength of the radiation. The peak wavelength (color) of an object (in nanometers) is related to temperature:
λ = 2.9X106 / T
Again, temperature must be expressed on the Kelvin scale. Wien’s Law therefore states that as an object gets hotter, it will glow brighter and, as its peak wavelength shortens, its color will shift toward the blue end of the spectrum and the average energy of the photons emitted increases. Very hot objects, such as the gases being pulled into the gravity well of a black hole, are heated to the point where their peak emissions are in the X-ray portion of the spectrum!
 Wien's Law states that the peak wavelength emitted by a blackbody varies with Temperature.
Wien's Law states that the peak wavelength emitted by a blackbody varies with Temperature.
4.2.3 Thermodynamics
The laws of thermodynamics govern the transfer of heat from one system to another. Heat tends to move from hotter objects to cooler ones, until both systems are in thermal equilibrium, in which their temperatures are equal. The First Law of Thermodynamics, also known as the Law of Conservation of Energy states that the total amount of energy in a closed system is always conserved. These means we can never get free energy. In order to perform any work, such moving an object or moving objects around, requires energy. We can change the from of energy, such as from potential energy to kinetic energy (Chapter 3) or we can transfer energy from one system to another, but it can never be created or destroyed. We can never get more energy out of a system than we put into it.
The Second Law of Thermodynamics or, the Law of Entropy, states that the entropy, or disorder of a system, always increases, so as energy changes forms, the amount of heat energy tends to increase. This means that in any energy system, some energy will be lost to waste heat. So, the amount of energy or useful work we can get out of a system will always be less than amount we put into it. The Law Entropy is universal in that the amount of entropy in the universe is steadily increasing. We can decrease entropy locally, as living organisms do when they use energy from the Sun to build complex molecules, but only by increasing the overall entropy. Eventually, everything runs down. Any energy conversion system, whether a living organism or a car’s engine, can only continue running so long as additional energy is put into it. Even so, parts wear out and need to be replaced over time. Likewise, even the Sun will run out usable hydrogen to fuse into helium and die. Even the entire universe, assuming it does not collapse into a “Big Crunch” (the opposite of a Big Bang), will eventually reach a state of maximum entropy where all usable energy will be dispersed and no systems will be able to function. Astronomers call this the Heat Death of the Universe. Do not get too worried about it. Scientists predict the Sun will not run out of hydrogen for another four or five billion years and the Heath Death is hundreds of trillions of years after that. In the meantime, the Second Law also dictates that it is impossible to remove all the heat energy from a system and therefore, it is impossible to reach absolute zero.
Heat can be transferred from one system to another by one of three ways. The first is conduction, which heat is transferred from direct contact between two systems, such as placing a pot on top of a stove burner. The movement of liquids or gases can also transfer heat through convection, such as air currents absorbing heat from Earth’s surface. Since warm air is less dense than cooler air, it rises (this is how a hot air balloon rises). As the warm air rises, the heat is dispersed into the upper troposphere. The air cools, becomes denser, and sinks back down to the surface. Radiation is the transfer of heat through electromagnetic radiation without any contact between materials. This is how energy from the Sun travels to the Earth.
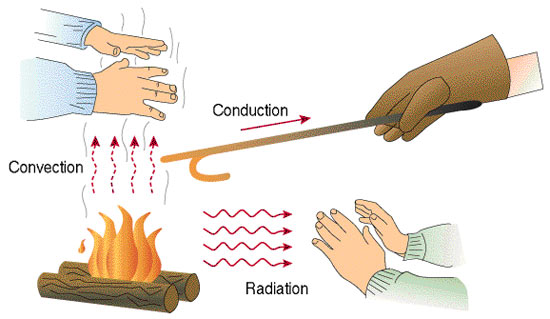 Heat is transmitted by three methods: Radiation, conduction, and convection.
Heat is transmitted by three methods: Radiation, conduction, and convection.
https:/commons.wikimedia.org/wiki/File:Heat-transmittance-means1.jpg;