4.3: Atomic Theory
( \newcommand{\kernel}{\mathrm{null}\,}\)
4.3.1 Early Atomic Theory
Ancient philosophers pondered the nature of matter for thousands of years. Many held that all matter was made up of four primal substances or elements, such as air, earth, fire, and water. Under this model, all matter was made up of different combinations of these four elements. In contrast, the Greek philosopher Democritus, held that matter was made up of tiny particles. He called these particles atoms, from the Greek word for “inseparable.” Democritus argued that any object could be cut into smaller parts but eventually, if we kept cutting, we would reach a tiny particle that could no longer split into smaller parts. This fundamental particle was thus, inseparable.
For thousands of years, philosophers favored the idea of primal elements over Democritus’ atomic theory, but by the late nineteenth century, physicists had concluded from observation that Democritus had been right all along. Matter was indeed made of tiny, inseparable particles.
But then, in 1900, Joseph John Thompson discovered the electron, a particle with a negative charge and that was lighter than the lightest known atom. Suddenly, atoms no longer seemed so “inseparable” at all, as there appeared to be a particle smaller and more fundamental than the atom. At first, physicists modeled atom as a mass of positive charges with negatively charged electrons embedded in it, like plums embedded in a pudding.
Eventually, however, other particles were discovered, such as the alpha particle, which we now know is a helium nucleus, containing two protons and two neutrons. Ernest Rutherford’s experiments with alpha particles revealed that, instead a “plum pudding” model, most of the atom’s mass was concentrated in a tiny point in the center, a nucleus containing protons and neutrons. Most of the atom, in fact, was empty space! Rutherford concluded that electrons must orbit the nucleus much like planets orbit the Sun.
 Rutherford's Atomic Model
Rutherford's Atomic Model
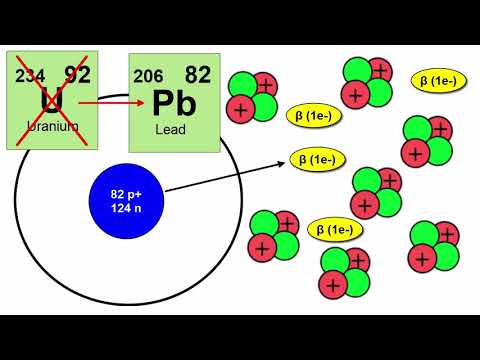
In Rutherford’s model, the nucleus of an atom contains two particles: positively charge protons and neutrons, which lack any charge. The number of protons in the nucleus, known as the atomic number, determines what element the atom is. The lightest atom, hydrogen, has an atomic number of one and therefore, only has one proton in the nucleus. The heaviest naturally occurring element, uranium has ninety-two protons in its nucleus, giving it an atomic number of ninety-two. Today, an element is defined as a substance that cannot be chemically broken down into any other substances. Instead of just four elements, the modern periodic table contains ninety-two naturally occurring elements and over twenty-five elements that have been produced artificially in nuclear reactors and particle accelerators. The total number of protons and neutrons in the atom is the atomic mass. Some atoms of the same element may have differing numbers of neutrons, and therefore, have different atomic masses. Such atoms, called isotopes, have slightly different masses and some are unstable, undergoing radioactive decay into lighter, most stable atoms. Isotopes of the same element are designated with a superscript of their respective atomic mass, for example, 12C and 14C, being two isotopes of carbon. Atoms can form bonds with each other by exchanging or sharing one or more orbital electrons. Two or more atoms bound together form a molecule. Molecules made of two or more different elements, such as water (H2O) or carbon dioxide (CO2) are called compounds.
 Radioactive atoms emit three kinds of radiation: Alpha, Beta, and Gamma.
Radioactive atoms emit three kinds of radiation: Alpha, Beta, and Gamma.
https:/www.needpix.com/photo/1315702/alpha-beta-gamma-radiation;
 A water molecule is an example of a compound.
A water molecule is an example of a compound.
Matter can exist in of four physical states. Three of those states are familiar to us: solid, liquid, and gas. The fourth state, plasma, consists of ionized gases, that is, gaseous atoms that have been stripped of their electrons. The state a substance exists in depends on the nature of the substance, its temperature, and pressure. For example, the atmospheric pressure on Mars is too low to keep water in a liquid state. A bottle of liquid water on Mars would quickly freeze solid, although solar radiation may cause some of the ice to sublimate, that is, turn directly from solid to the vapor phase. On the other hand, Venus has an atmospheric pressure on the surface that is ninety times that of Earth at sea level, more than enough to keep water in a liquid form. However, the temperature on Venus is far too high for liquid water and any water on the surface would boil away in an instant. Of the terrestrial planets, only the Earth has the right combination of temperature and pressure for liquid water to exist on the surface.
 A plasma globe produces a plasma, fluid of charged particles.
A plasma globe produces a plasma, fluid of charged particles.
https:/commons.wikimedia.org/wiki/File:Plasma_globe_60th.jpg;
At very high temperatures, such as those found on the Sun, gases may undergo ionization, in which their atoms are stripped of their electrons, becoming plasma. Another change in matter of interest to planetary astronomers is dissociation, in which radiation breaks the chemical bonds in molecules, separating them into their individual atoms. Dissociation often occurs in the upper atmospheres of the terrestrial planets.
4.3.2 Bohr Atomic Model
Able to explain how atoms behave and form bonds, Rutherford’s planetary model seemed to explain the observed behavior of atoms.
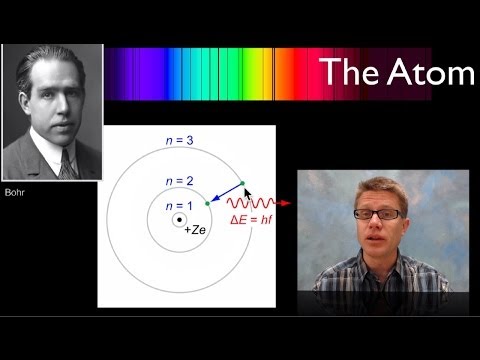
However, Niels Bohr soon discovered a problem with the standard planetary model. He knew that an accelerating charge, such as an electron moving in a circular orbit, emits electromagnetic radiation. As it did, it would lose energy and spiral into the nucleus. It seemed Rutherford’s planetary model was unstable and could not explain how electrons remained in their orbits. Bohr also noted that electrons could only exist in certain energy states as they orbited an atom. For example, an electron could exist at the lowest energy state, the ground state, and at certain excited states, but it could never exist in an orbit in between these states. The electron remained in the ground state until it absorbed a photon of exactly the amount of energy needed to excite it to one of the higher states. It could then fall back to the ground state by emitting a photon of the exact same energy. However, if a photon with an intermediate energy, such as between the energy of the ground state and the first excited state, nothing happened. The electron could not absorb any photon except those whose energies were an exact match of the difference between its current state and an upper, excited state. Likewise, it would only emit a photon of energy equal to the difference between its current state and the lower state. Electrons in an excited state can fall back to the ground state directly, such as from the third excited state to the ground state, or as a cascade, dropping from the third excited state, to the second, to the first, and finally back to the ground state.
It is important to note that electrons do not move up or down from one state to another as a smooth transition, like a ball rolling down a ramp. Instead, they behave more like steps on a ladder. The electron can be on the bottom state or any of the higher states, but it cannot exist in any space in between two states, just as your foot could not rest in any space between two rungs on a ladder. The Bohr Model of the atom thus defines electron orbits as specific, quantized energy states instead of any of a spectrum of orbits like planets. The energy states that electrons can occupy depend on the number of protons in the nucleus and the number of electrons orbiting it. As a result, the wavelengths of light that the electrons can emit or absorb are unique to each element.
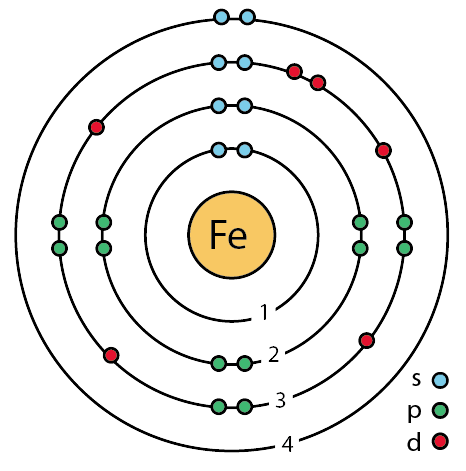_enhanced_Bohr_model.png?revision=1) Bohr model of an iron atom.
Bohr model of an iron atom.
 In the Bohr model, a photon of a specific energy is absorbed by an electron, causing it to jump to a higher energy state. As the electron falls to a lower energy state, a photon of the same energy is emitted.
In the Bohr model, a photon of a specific energy is absorbed by an electron, causing it to jump to a higher energy state. As the electron falls to a lower energy state, a photon of the same energy is emitted.