4.4: Kirchhoff’s Laws
- Page ID
- 30875
4.4.1 Spectroscopy
Isaac Newton demonstrated that white light can be separated into its component colors. Pure white light contains the six colors of the visible spectrum: red, orange, yellow, green, blue, and violet. A spectroscope consists of a prism to separate colors and then projects them onto a screen or detector for analysis. Astronomers use spectroscopes to examine the light coming from stars and other bodies. A curious thing happens, though, when we examine the light coming from, say, a cloud of thin, hot gas. Instead of seeing a continuous spectrum, we see a series of discrete lines. These emission lines are produced by electrons dropping from an excited state to a lower state. The wavelengths emitted, as explained by the Bohr Model, are unique for each element. As a result, by examining the emission lines, we can determine what elements the cloud of is made.
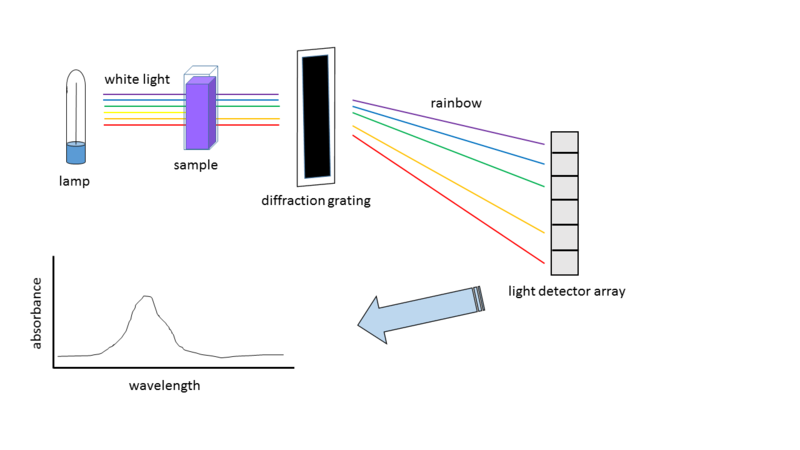 A spectroscope analyzes light by using a prism to split it into its various colors.
A spectroscope analyzes light by using a prism to split it into its various colors.
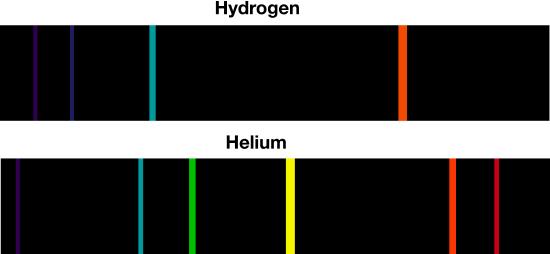 Atomic emission spectrum of helium.
Atomic emission spectrum of helium.
If we examine the light after it passes through a cool gas, we see what appears to be a near-continuous spectrum, but with several blank lines in which specific wavelengths of light have disappeared. These absorption lines are the result of electrons absorbing light and jumping from a lower state to a higher energy state. The specific wavelengths in the absorption lines of an element are the same as the wavelengths in the emission lines of the same element. Thus, we can identify elements by either their emission or absorption spectra.

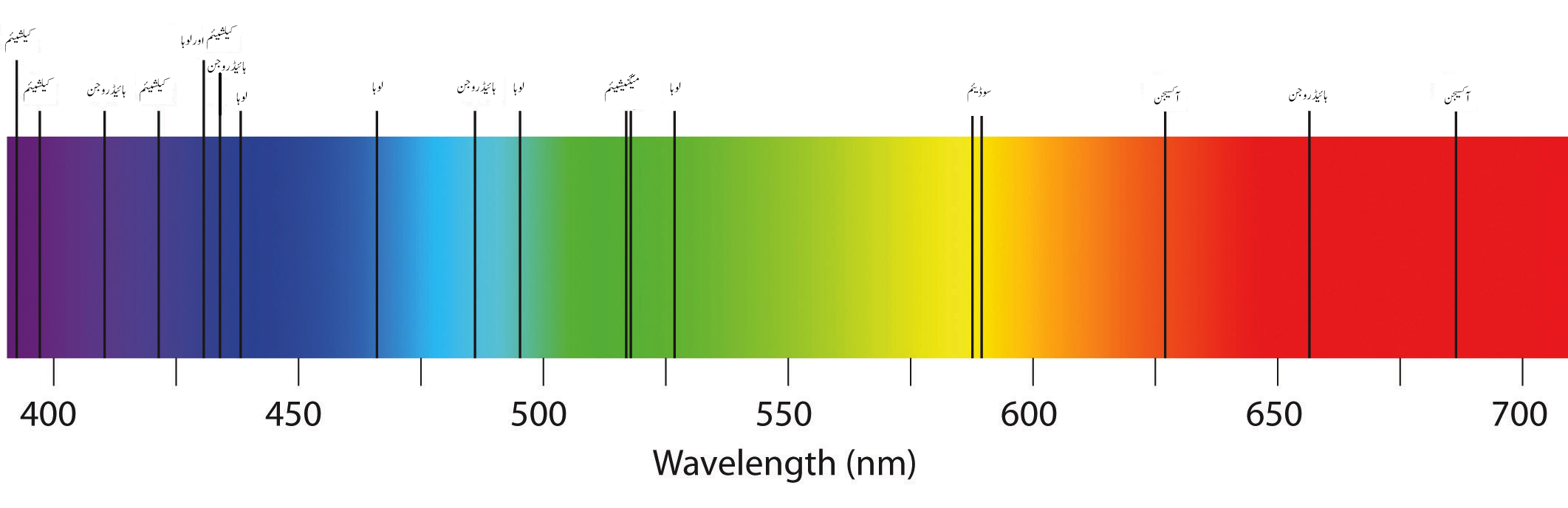 The absorption spectrum of a few elements.
The absorption spectrum of a few elements.
4.4.2 Kirchhoff's Laws of Radiation
Bohr developed his model of the atom in part by examining the emission and absorption spectra of hydrogen. Hydrogen is the simplest of the atoms as it has only one electron orbit the nucleus. Multielectron atoms have more complex spectra. Molecules have even more complex spectra, such as the spectra for molecular hydrogen, consisting of two hydrogen atoms bonded together, are more complex than that of a single hydrogen atom. However, all these various spectra behave in predictable fashion, so each acts a definitive “fingerprint” that can be used to identify the element or compound present.
As noted above, whether a substance produces an emission or an absorption spectrum depends in part on its temperature as well as its state and density. Kirchhoff’s Laws formally describe what kinds of spectra emitted as follows:
1. A hot solid, liquid or gas, under high pressure, gives off a continuous spectrum.
2. A hot gas under low pressure produces a bright-line or emission line spectrum.
3. A dark line or absorption line spectrum is seen when a source of a continuous spectrum is viewed behind a cool gas under pressure.
Kirchhoff’s Laws have proven to be useful in identifying the composition and temperature of everything from planetary atmospheres, stars, and interstellar nebula. But they do not tell us anything about the motion of these objects. For that, we will need to consider another property of electromagnetic waves, the Doppler effect.
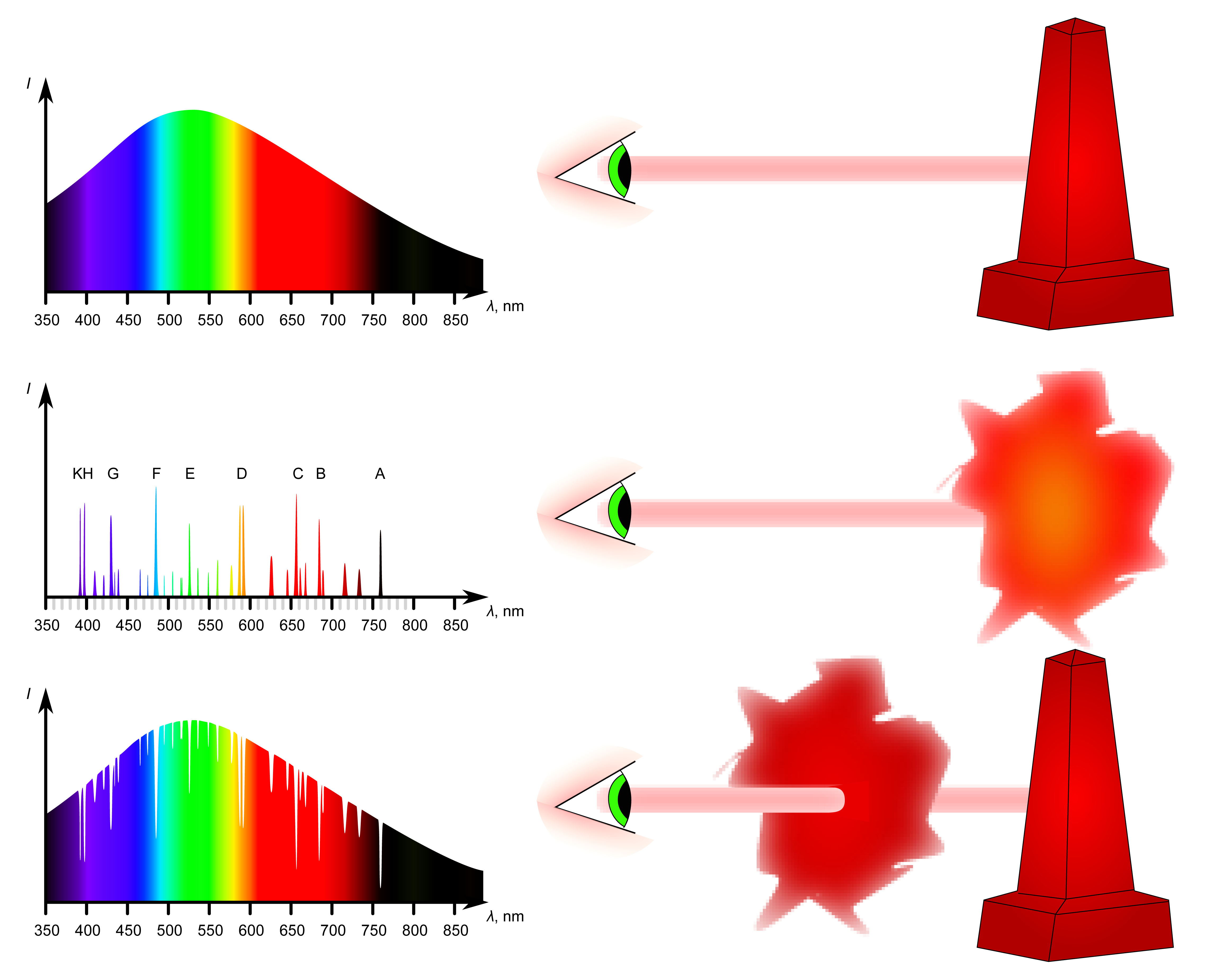 Kirchhoff's Laws describe light emitted from a luminous solid, a hot gas, and a cool, diffuse gas.
Kirchhoff's Laws describe light emitted from a luminous solid, a hot gas, and a cool, diffuse gas.

