15.2: Astrobiology
( \newcommand{\kernel}{\mathrm{null}\,}\)
15.2.1 Extremophiles
The discipline of astrobiology involves the study of the origins, evolution, distribution, and future of life in the universe. It may seem strange to have a discipline devoted to studying something that has yet to be discovered. After all, we only have one place where life is confirmed. However, we can study life in locations on Earth that were more extreme than the norm. We have found life in places on Earth that we would not have expected them to be. These organisms are called extremophiles. These organisms live in environments considered too extreme for most living organisms. However, for the extremophiles, these environments perfectly suited for them and the “normal” environment most other organisms live are the extremes.
There are different categories of extremophiles, including:
- Themophiles: Bacteria and archaea that thrive in extreme temperatures. These are often found in the scalding waters of geyser such as Old Faithful in Yellowstone National Park.
- Psychrophiles: These extremophiles that thrive in temperatures lingering around the freezing point of water. Often, they are found in pools of water buried under sheets of ice in places like Antarctica.
- Acidophiles: Microorganisms that thrive in pH at lowers at or below 2. They can be found in deep-sea vents, volcanic areas, areas subjected to acids draining from metal mines, or even in the stomachs of animals.
- Alkaliphiles: The opposite of acidophiles, alkaliphiles thrive in environment of pH, often in ranges of 8.5 to 11. These environments include soda lakes found in Yellowstone or other locations where the alkaline compounds can accumulate in water.
- Radioresistant: These microbes are resistant to high levels of ionizing radiation or UV radiation. Their ability to resist and repair the damage radiation causes to DNA has enabled them to survive even in the coolant waters of nuclear power plants.
- Barophiles: These can resist the intense pressures found in the deep waters of places like the Mariana Trench
- Halophiles: These microbes can survive in environments with extreme levels of salinity, such as the Great Salt Lake or the Dead Sea.
- Xerophiles: These include fungi and yeast that have adapted unique features that allow them to survive extreme desiccation.
 The Mammoth Hot Springs are home to thermophiles.
The Mammoth Hot Springs are home to thermophiles.
https:/commons.wikimedia.org/wiki/File:MAMMOTH_HOT_SPRINGS_-_EXTREMOPHILES.jpg;
 The waters in the Rio Tinto have a pH of about 2.2 and are home to acidophiles.
The waters in the Rio Tinto have a pH of about 2.2 and are home to acidophiles.
https:/commons.wikimedia.org/wiki/File:Rio_Tinto_water,_pH_2,2.jpg;
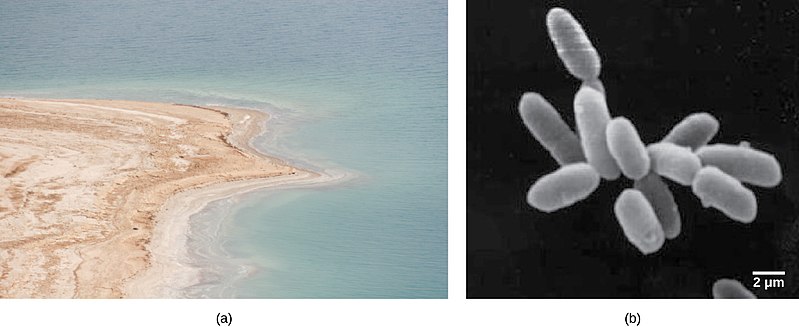 Halophiles thrive in environments with high salt content like the Dead Sea.
Halophiles thrive in environments with high salt content like the Dead Sea.
https:/commons.wikimedia.org/wiki/File:Figure_22_01_05.jpg;
15.2.2 Tardigrades
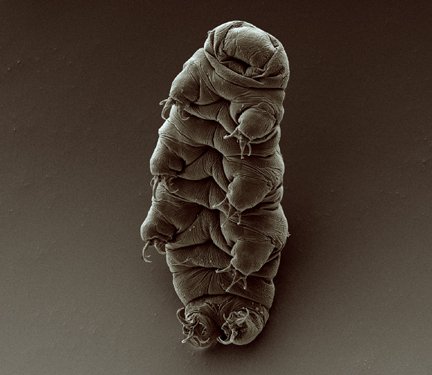 Adult tardigrade.
Adult tardigrade.
https:/commons.wikimedia.org/wiki/File:Adult_tardigrade.jpg;
Most extremophiles are single-celled organisms like bacteria, archaea, or protozoa. However, there is one multicellular animal that may be the champion of extremophiles, the Tardigrade or water bear. Tardigrades can survive in extreme environments that would kill almost any other animal. These include:
- Temperature: Tardigrades can survive have been known to survive:
- A few minutes at 151 °C (304 °F)
- 30 years at −20 °C (−4 °F)
- A few days at −200 °C (−328 °F; 73 K)
- A few minutes at −272 °C (−458 °F; 1 K)
- Pressure: They can withstand the extremely low pressure of a vacuum and also very high pressures, more than 1,200 times atmospheric pressure. Tardigrades can survive the vacuum of open space and solar radiation combined for at least 10 days.
- Dehydration: The longest that living tardigrades have been shown to survive in a dry state is nearly 10 years, where they go into a dormant state until rehydration.
- Radiation: Tardigrades can withstand 1,000 times more radiation than other animals.
Tardigrades are the first known animal to survive in the harsh environment of space. In September 2007, dehydrated tardigrades were taken into low Earth orbit on the FOTON-M3 craft. For 10 days, groups of tardigrades were exposed to either the hard vacuum of outer space or vacuum and solar UV radiation. After being rehydrated back on Earth, over 68% of the subjects protected from high-energy UV radiation revived within 30 minutes following rehydration.
15.2.3 Commonalities of Life
Because it is doubtful that any alien planet will exactly replicate the conditions on Earth, studying extremophiles can give us an idea of the range of environments in which life can survive.
In studying life on Earth, we do have some commonalities even among the extremophiles. All organisms use organic molecules, that is, molecules based on carbon. Carbon’s ability to form four distinct bonds and form long chains or other shapes of flexible molecules has made it the basis for all life. Organic molecules are used for all aspects of biochemistry, from structural material (proteins), energy storage (lipids, carbohydrates), catalysts (proteins), and encoding genetic information (DNA and RNA).
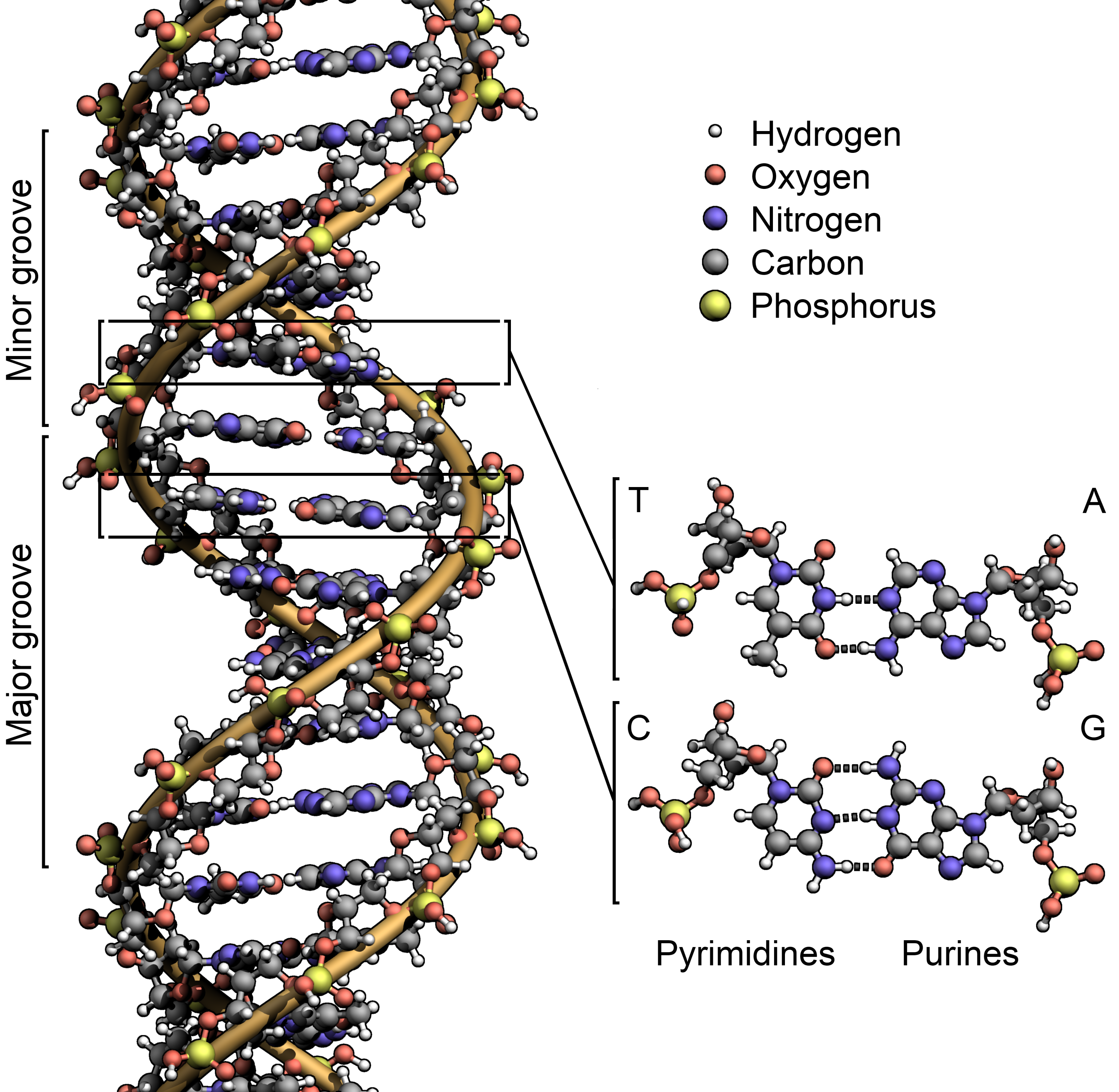 The DNA double helix.
The DNA double helix.
In addition, life as we know it requires water as the medium in which to conduct the chemical reactions necessary for life. Water is a polar molecule. Even though the molecule as a whole is neutral, the oxygen atom exerts a stronger pull on the electrons than the two hydrogen atoms. These means the shared electrons spend more time around the oxygen atom, giving it a slight negative charge while the hydrogen atoms have a slight negative charge. This polarity gives water is relatively high freezing temperature and makes it a powerful solvent. More substances dissolve in water than any other fluid. This makes water ideal as the medium for organisms to conduct their chemical reactions as well as for cells to use to take in nutrients and expel wastes.
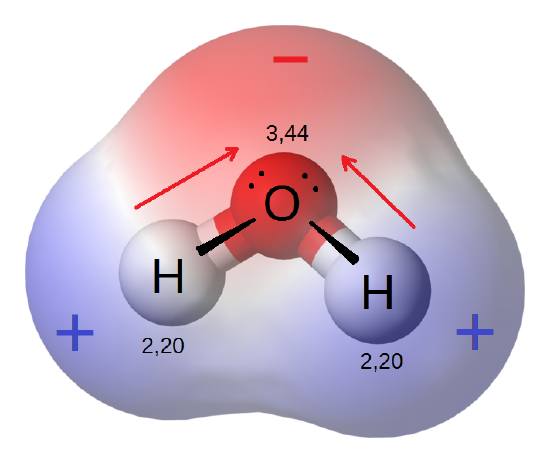 Water is a polar molecule.
Water is a polar molecule.
Finally, all life on Earth has the same chirality or “handedness.” The amino acids used to build proteins all share the same left-handed orientation. Amino acids with right-handed orientation are mirror images of their left-handed counterparts. Molecules that are mirror images of each other are called isomers. Scientists are not sure why left-handed amino acids came to be the basis for life on Earth. It is possible that on other planets, right-handed amino acids may be utilized.
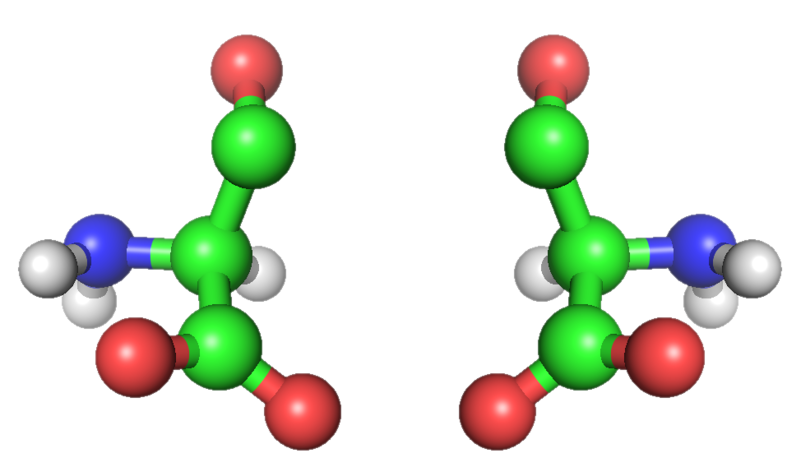 Left and right-handed molecules are mirror images of each other.
Left and right-handed molecules are mirror images of each other.
So far, we have discussed the chemistry of life as we know it on Earth. What about alternative biochemistries? One staple of science fiction is life based on silicon instead of carbon. Silicon does have similar chemical properties as carbon, including the ability for up to four bonds with other atoms. However, silicon tends to form more rigid, crystalline molecules instead of the flexible organic molecules that carbon does. In addition, silicon is less likely to form complex chains like carbon does without large amounts of energy. In addition, some astrobiologists point to the fact that silicon is more abundant than carbon in Earth’s crust. So, if silicon-based life were possible, it would have made use of the abundant silicon instead of the rarer carbon.
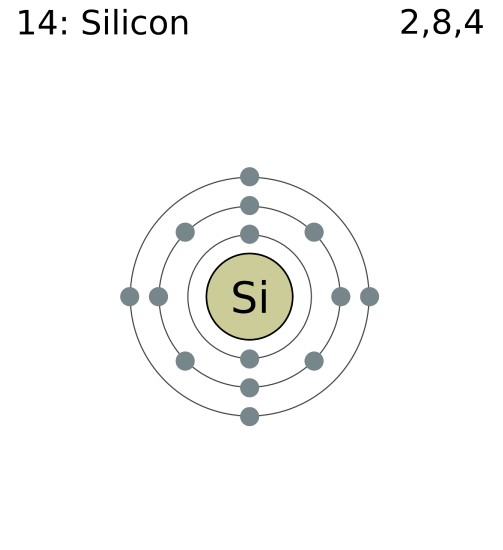 Silicon has some similar chemical properties to carbon.
Silicon has some similar chemical properties to carbon.
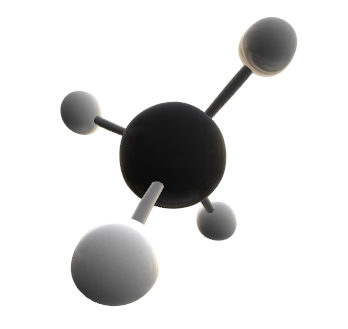 Methane and other hydrocarbons are abundant on Titan.
Methane and other hydrocarbons are abundant on Titan.
Methane_Molecule_3D_X.jpg https://commons.wikimedia.org/wiki/F...ecule_3D_X.jpg
Another possibility could be using fluids like ammonia or methane as the solvent instead of water. However, these fluids have much lower boiling temperatures than water, so any chemical reactions occurring in liquid methane or ammonia would happen very slowly, making active organisms like animals unlikely. In addition, methane lacks the polarity of water, making it a far less effective solvent.






