3.3: The Fluid Phase
- Page ID
- 869
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Cell membranes are composed of many different types of lipids, including saturated and unsaturated phospholipids, cholesterol, and fatty acids. Membranes in functional condition are predominantly comprised of fluid phase lipid bilayers. Maintenance of membrane fluidity is crucial for integrity and functionality. However, phase coexistence is normal since the membrane is comprised of many different lipid molecules. The addition of sphingolipids increases the possibility of gel (solid) phase coexisting with fluid (liquid) phase. This is due to sphingolipids containing long, saturated acyl chains that cause them to pack tightly in the membrane, resulting in very limited room for lateral diffusion. Some damaged cells undergo lipid phase transition from fluid phase to gel phase in the membrane, which results in fatal problems and cell death. This also occurs when the cell ages, as lipids in the fluid phase emerge in the gel phase and vice versa. Physiological and metabolic changes occur as a result of the changes in lipid composition.
The plasma membrane is a semi-permeable bilayer barrier that separates the interior of the cell from the exterior and maintain a distinct metabolism in separate cellular compartments. Cell organelles within the intracellular environment also rely on the presence of membranes and the cytoskeleton for structure and function. The functionality of the bilayer is carried out by proteins and determined by the physical properties, a great deal of which depends on the lipid conformation of the membrane.
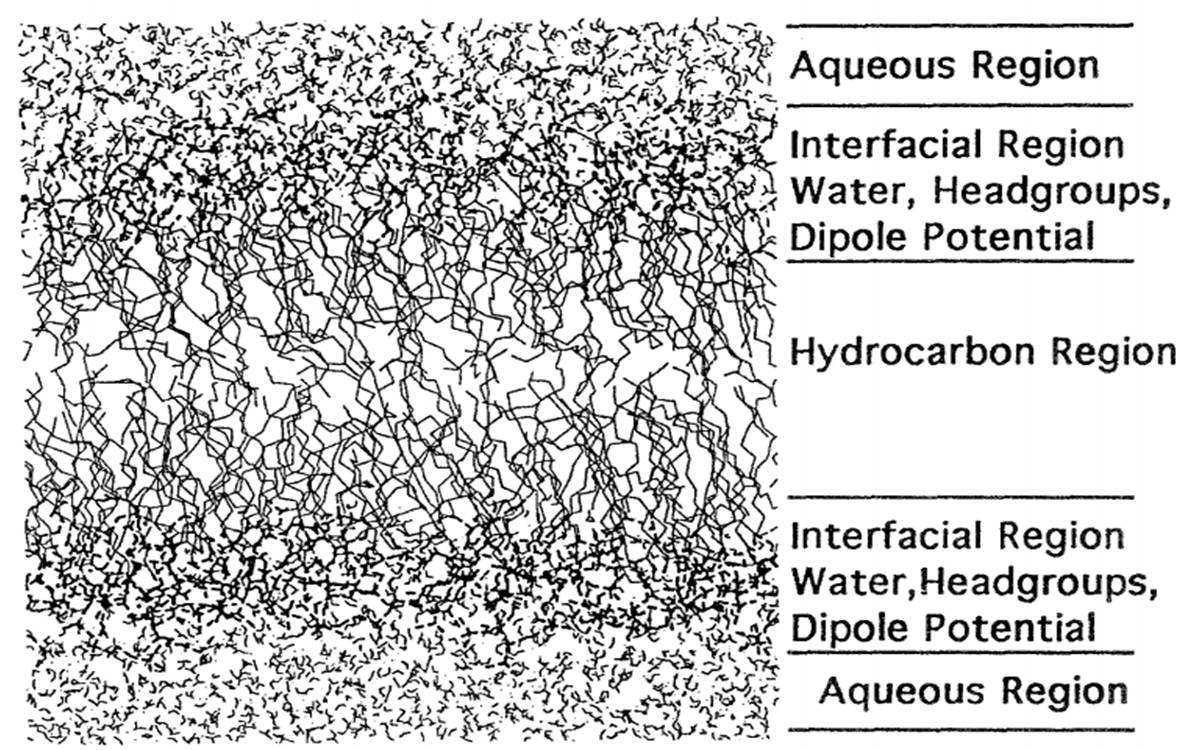
The bilayer fluid domain consists of a liquid-ordered phase (Lo) and a liquid-disordered phase (Ld). Though, it should be noted that, for practical applications, the heterogeneity of bio-membranes cannot be simplified to mere differentiation of coexisting lipid domains. Protein interactions must be taken into consideration with regard to heterogeneity thermodynamics, as they are significant to cell metabolism.

II. Fluid phase lipid bilayer membrane
II.a. Temperature Changes
At a given temperature, a short-tailed lipid has more fluidity than a long-tailed counterpart due to packing characteristics. A decrease in temperature results in loss of membrane fluidity due to the lipid acyl chains’ formation of greater numbers of Van der Waals interactions. Likewise, an increase in temperature results in greater membrane fluidity as Van der Waals interactions are disrupted and broken. When the environment reaches the critical temperature \(T_m\), and the membrane is transitioning from gel phase to fluid phase:
- Rotational isomers emerge in the hydrocarbon chains
- Rapid lateral diffusion increases among the lipid molecules
- The bilayer membrane expands, causing a decrease in electrostatic free energy
Shorter chain lengths in saturated lipids have decreased \(T_m\) in comparison to longer chain lengths. As the number of carbons in the lipid alkane chains increases, so does the gel to liquid phase transition temperature. The \(T_m\) of dipalmitoylphosphatidylcholine (DPPC) and 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) is approximately 41˚C and 54˚C, respectively.3 Usually, there exists a hysteresis curve between cooling and warming.
The enthalpy change \(ΔH\), or heat absorbed at the end of phase transition, can be described by the following equation:7
\[ΔH = T_m \times ΔS.\]
where \(ΔS\) is entropy change and \(T_m\) the critical temperature (transition temperature). \(ΔH\) may be also be denoted as the sum of a non-electrostatic value, \(ΔH^o\), and an electrostatic value:7
\[ΔΓ = Γ (fluid) - Γ (ordered). \]
Thus, considering electrostatic effects, \(ΔT_m\) can be described by:7
\[ΔT_m = T_m-T_m^o = \dfrac{ΔΓ}{ ΔS}\]
where
\[T_m^o = \dfrac{ΔH˚}{ΔS}\]
is the value of \(T_m\) in the absence of electrostatic effects.
Phase behavior is determined by degree of tail saturation and headgroup conformation. Bio-membranes are composed of lipids with various degrees of saturation. Poly-unsaturation may reach as high as six double bonds per chain. Unsaturated and short-chain lipids have decreased \(T_m\), while saturated and long-chain lipids have increased \(T_m\).
Phase behavior and \(T_m\) are affected by many factors:8
- Vesicle size (determines the thermodynamic limit)
- Acyl chain length
- Presence, number, and positioning of double bonds. Double bonds decrease \(T_m\) and allow lipids to contain longer chains.
- pH
- Hydration status (fluidity)
- Cholesterol
- Addition of a second lipid
- Alkane chain order
Lipid phase behavior depends on the strength of the Van der Waals interactions between adjacent lipid molecules. Van der Waals interactions are affected by lipid tail length; long-tailed lipids have a greater area for potential interaction and therefore give rise to stronger Van der Waals interactions and decreased lipid mobility. Consequently, the \(T_m\) is increased and the lipid shifts away from the fluid phase and toward the gel phase.8 Van der Waals interactions also contribute to the degree of saturation of the membrane. Unsaturated lipids (lipids containing double bonds in the tails) disrupt membrane packing and render the membrane more hydrophilic.
At physiological temperatures, bilayer membranes are usually in fluid phase. The relative fluidity of individual lipid molecules, and subsequently the lipid membrane, is dependent on temperature. At a certain temperature (Tm), the lipid will undergo phase transition from the gel to the fluid phase. In fluid phase, lipid molecules undergo rapid diffusion and move freely within the two dimensional plane occupied by the membrane. Hence, they may easily traverse long distance within the plane over given periods of time. Even so, there exist constraints on lipid motion due to pressure from bulk lipids at different areas of the bilayer.
The gel phase, in contrast, is characterized by rigidity and limited mobility. Unlike in the fluid phase, lipids in the gel phase do not exchange positions with one another or “flip flop”. Because of this, gel phase bilayers are unable to patch small holes made in the membrane.
II.b. Cholesterol
The addition of cholesterol to a fluid phase bilayer decreases the bilayer’s permeability to water and coagulates the bilayer, disrupting local packing and increasing bilayer rigidity. This is due to cholesterol intercalating between alkane tails of lipid molecules. As it occupies the free space, it decreases the flexibility and elasticity of surrounding lipid chains. Consequently, the lateral diffusion coefficient effectively decreases. Possibly, cholesterol mediates the solubilization of the bilayer until the entire surface comprises of a fluid phase with high cholesterol content.4 However, in conditions below melting temperatures, addition of cholesterol to phosphatidylcholine membranes results in greater membrane fluidity.
II.c. Ion Concentration and pH
The “Gouy-Chapman theory” predicts a decrease of transition temperature with increasing charge density.7 An old study found that divalent cations such as Mg2+ and Ca2+ increase \(T_m\) via charge neutralization while monovalient cations such as Li+, Na+, and K+, decrease \(T_m\) or spontaneously fluidize the bilayer at certain temperatures.7 The pH changes required to change the \(T_m\) were minimal, i.e. a change from pH 7 to pH 9 was adequate to decrease \(T_m\) by 20˚C.7
III. Methods of Study
Gel and fluid phases of bilayers may be differentiated via electron microscopy, X-ray diffraction, and magnetic resonance spectroscopy. In addition, epi-fluorescence microscopy of lipid systems utilizes fluorescent probes to differentiate between gel and fluid phase. The efficacy of this technique is optimized with supplementation with photo-bleaching recovery experiments.
Structural information of the fluid phase is difficult to acquire, notably for liquid-disordered phases. Crystallography is an inadequate method as the diffraction signals caused by the fluid phase result from liquid crystals rather than solid. This makes it difficult to form an accurate image of the bilayer phase. Fully hydrated fluid phase bilayers have inherent fluctuating and unstable stacking properties. Partially hydrated fluid phases are composed of nonlinear structures induced by bilayer forces as the structures shift closer, rendering data obtainment difficult. A possible option is to use unilamellar vesicles rather than liquid crystalline arrays. With this method, scattering data obtained from X-rays may be used for analysis.
There are three general scales at which lipid bilayer structure is measured:2
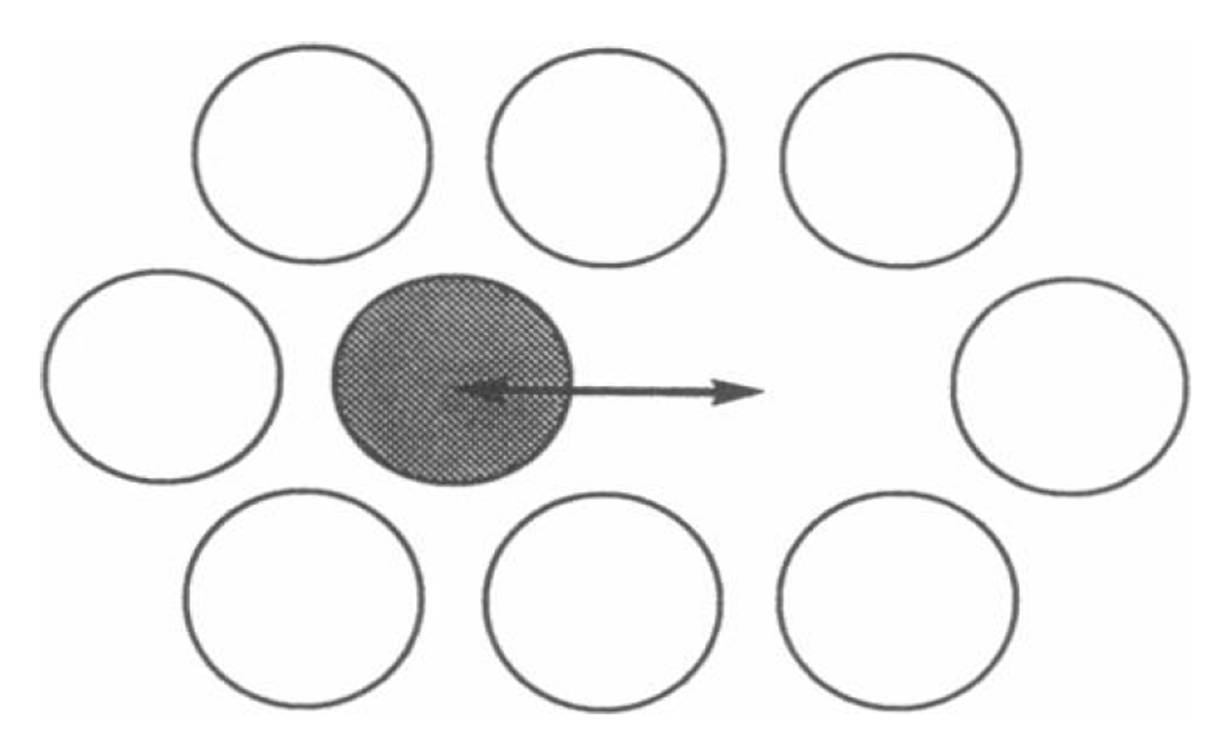
- Short range diffusion over distances roughly the length of the diameters of two lipid molecules. Methods such as quasi-elastic neutron scattering and small angle neutron usage are utilized. Because the measurement is taken over short time periods (< 10^-9 seconds), the actual distance traveled by lipid particles is no more than the vibration or movement of the particle in its immediate environment (see Figure \(\PageIndex{3}\)).
- Intermediate range diffusion over distances of several lipid diameters.
- Long range diffusion over several micrometers using methods such as fluorescence recovery after photo-bleaching (FRAP) and magnetic resonance techniques. These techniques measure sum translational diffusion and "effective displacement" over large ranges (Figure \(\PageIndex{3}\)).
Bilayers can be visualized using fluorescence microscopy. In fluorescence microscopy, a sample is excited using one wavelength of light and emitted in another wavelength. Thus, fluorescent molecules, which have matching excitation and emission properties, are the only molecules that can be visualized.
Electron microscopy is another method to visualize bilayers. In contrast with fluorescence microscopy, electron microscopy delivers a higher resolution image because concentrated electrons are used in place of a wavelength of light. Since electrons have much shorter wavelengths compared with light photons, a greater resolution may be achieved. This is conducive to studying the structure of very small samples.
The issue with utilization of different techniques is that they may be sensitive to different facets of bilayer thickness. Neutron scattering senses the high contrast between protonated lipid and deuterated water molecules; the technique uses this contrast to define overall bilayer thickness (D8), known as “Luzzati thickness”3. In contrast, the thickness calculated by X-ray scattering is the distance measured between the peaks in the electron density profile, which corresponds to the distance between the lipid headgroups (DHH).
Although neither neutron scattering or X-ray scattering on its own can be used to accurately depict bilayer structure, a scattering density profile (SDP) model combines X-ray and neutron scattering data to sum up the area “A” per lipid along the bilayer surface. Subsequently, bilayer thickness is compared to A via volume comparisons.3 Depending on hydration status of the membrane, water molecules may also intercalate into the headgroup region and influence thickness calculation.
Furthermore, “lipid bilayer thickness” is a relatively loose term that may be described in various ways. In literature, hydrophobic thickness (DC) may be used in circumstances wherein a membrane protein is affected by hydrophobic mismatch, in which case the bilayer thickness is defined as the hydrocarbon acyl chains and neglects the presence of water. However, as aforementioned, water molecules may be present in the headgroups, so the thickness (DH) may instead be somewhat arbitrarily taken from headgroup conformation and steric thickness.3
References
- Chiu SW, Clark M, Balaji V, Subramaniam S, Scott HL, Jakobsson E. "Simulation of a Fluid Phase Lipid Bilayer Membrane: Incorporation of the Surface Tension into System Boundary Conditions". Molecular Engineering 5: 45-53, 1995.
- Vaz WLC, Almeida PF. "Microscopic versus macroscopic diffusion in one-component fluid phase lipid bilayer membranes". Biophysical Journal Volume 60:1553-1554, 1991
- Kučerka N, Nieh MP, Katsaras J. "Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature". Biochimica et Biophysica Acta 1808: 2761–2771, 2011.
- Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. "Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles". PNAS Vol 104 No. 9:3165-3170, 2007
- Kučerka N, Liu Y, Chu N, Petrache HI, Tristram-Nagle S, Nagle JF. "Structure of Fully Hydrated Fluid Phase DMPC and DLPC Lipid Bilayers Using X-Ray Scattering from Oriented Multilamellar Arrays and from Unilamellar Vesicles". Biophysical Journal Volume 88:2626–2637, 2005.
- Korlach J, Schwille P, Webb WW, Feigenson GW. "Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy". Proc. Natl. Acad. Sci. USA Vol. 96: 8461–8466, 1999.
- Trӓuble H, Eibl H. "Electrostatic Effects on Lipid Phase Transitions: Membrane Structure and Ionic Environment". Proc. Nat. Acad. Sci. USA Vol. 71, No. 1, pp. 214-219, 1974
- Faller, R. Lecture Notes. Univeristy of California Davis, 2014.

