7.P: Exercises
( \newcommand{\kernel}{\mathrm{null}\,}\)
- Calculate the energy-shift in the ground state of the one-dimensional harmonic oscillator when the perturbation
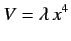 is added to
is added to 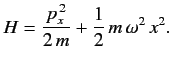 The properly normalized ground-state wavefunction is
The properly normalized ground-state wavefunction is 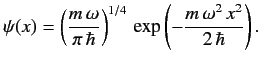
- Calculate the energy-shifts due to the first-order Stark effect in the n=3 state of a hydrogen atom. You do not need to perform all of the integrals, but you should construct the correct linear combinations of states.
- The Hamiltonian of the valence electron in a hydrogen-like atom can be written
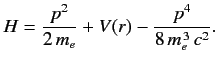 Here, the final term on the right-hand side is the first-order correction due to the electron's relativistic mass increase. Treating this term as a small perturbation, deduce that it causes an energy-shift in the energy eigenstate characterized by the standard quantum numbers n , l , m of
Here, the final term on the right-hand side is the first-order correction due to the electron's relativistic mass increase. Treating this term as a small perturbation, deduce that it causes an energy-shift in the energy eigenstate characterized by the standard quantum numbers n , l , m of 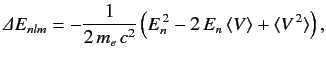 where En is the unperturbed energy, and α the fine structure constant.
where En is the unperturbed energy, and α the fine structure constant. - Consider an energy eigenstate of the hydrogen atom characterized by the standard quantum numbers n , l , and m . Show that if the energy-shift due to spin-orbit coupling (see Section 7.7) is added to that due to the electron's relativistic mass increase (see previous exercise) then the net fine structure energy-shift can be written
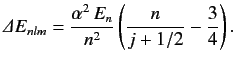 Here, En is the unperturbed energy, α the fine structure constant, and j=l±1/2 the quantum number associated with the magnitude of the sum of the electron's orbital and spin angular momenta. You will need to use the following standard results for a hydrogen atom: ⟨a0r⟩ =1n2, ⟨a20r2⟩ =1(l+1/2)n3, ⟨a30r3⟩ =1l(l+1/2)(l+1)n3. Here, a0 is the Bohr radius. Assuming that the above formula for the energy shift is valid for l=0 (which it is), show that fine structure causes the energy of the (2p)3/2 states of a hydrogen atom to exceed those of the (2p)1/2 and (2s)1/2 states by 4.5×10−5eV .
Here, En is the unperturbed energy, α the fine structure constant, and j=l±1/2 the quantum number associated with the magnitude of the sum of the electron's orbital and spin angular momenta. You will need to use the following standard results for a hydrogen atom: ⟨a0r⟩ =1n2, ⟨a20r2⟩ =1(l+1/2)n3, ⟨a30r3⟩ =1l(l+1/2)(l+1)n3. Here, a0 is the Bohr radius. Assuming that the above formula for the energy shift is valid for l=0 (which it is), show that fine structure causes the energy of the (2p)3/2 states of a hydrogen atom to exceed those of the (2p)1/2 and (2s)1/2 states by 4.5×10−5eV .
Contributors
Richard Fitzpatrick (Professor of Physics, The University of Texas at Austin)


