1.5: The Smallest Stuff- Particles, Atoms, and Molecules
( \newcommand{\kernel}{\mathrm{null}\,}\)
- You will know that matter is composed of atoms, which are composed of particles
Three students are studying for their upcoming exam.
Jue: I am confused by all these different particles. Atoms make up electrons and protons, is that right?
Keiko: No, electrons and protons make up atoms—atoms are bigger than either electrons or protons.
Lourdes: That’s right. Neutrons are part of atoms too.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
Our bodies, the air we breathe, and the planets and stars are all composed of atoms. An atom is composed of a dense central nucleus made up of protons and neutrons, which is surrounded by an electron cloud. Since the electron cloud is much larger than the nucleus, most of an atom is empty space. An atom is about 10⁻10 m in size, while the nucleus, at 10⁻15 m, is 100,000 times smaller. To put this into perspective, if the nucleus were half the length of an American football field, the size of an atom would be about the distance across the continental United States.
The type of atom, or chemical element, depends on the number of protons it contains—this is called its atomic number. Changing this number would mean changing the element and would require a nuclear reaction to do so. Figure 1.27 shows the Periodic Table of the Elements. It organizes all of the known elements, and this version gives the symbol for each element and its atomic number (number of protons) and atomic mass (number of protons plus neutrons, averaged over different forms of each atom called isotopes - see below). The mass of an atom depends almost entirely on the number of protons and neutrons; the electrons are so much lighter that their contribution to the mass is negligible. All known elements are shown in this table, including many that are familiar to you such as carbon (C), oxygen (O), and iron (Fe), and some that may be less familiar because they occur less frequently in nature, for example, lithium (Li) and beryllium (Be).

The atomic number of an atom determines its chemical properties, because each proton is paired with an electron orbiting the nucleus, and the outer electrons of an atom determine the atom’s chemistry. Since the positive electric charge of the proton has the same strength as the negative electric charge of the electron, the total charge of an atom is zero. In some cases, the atom can lose some of its electrons, or more rarely, gain extra electrons. In either case, the atom will have a net electrical charge and is then called an ion.
Two atoms with the same number of protons but a different number of neutrons are known as isotopes of the same element. For example, the most common form of hydrogen has just a single proton for a nucleus (atomic number 1, mass number 1). An isotope of hydrogen that has one proton and one neutron in the nucleus is called deuterium (atomic number 1, mass number 2). Both of these isotopes of hydrogen have a single electron orbiting the nucleus (whose negative electric charge balances the positive charge of the proton). As another example, helium usually has two protons and two neutrons (atomic number 2, mass number 4), but there is an isotope of helium that has two protons and one neutron (atomic number 2, mass number 3). Both of these isotopes of helium have two electrons. Isotopes of an element are often abbreviated with the element’s name or symbol and the mass number. For example, the isotope of helium with just one neutron is abbreviated helium-3 or 3He; the more common form of helium, used in helium balloons, is abbreviated helium-4 or 4He. In Figure 1.27, the mass number reported is a weighted average over all the different isotopes for that species. For this reason, the mass numbers shown are not integers.
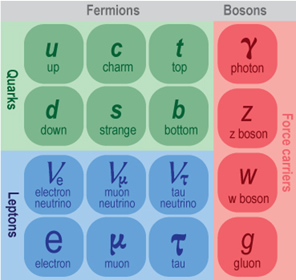
While we often think of atoms as the building blocks of matter, they are themselves composed of the subatomic particles protons, neutrons, and electrons. As it turns out, there is a series of more fundamental, or elementary, particles, from which all matter in the Universe is made. Protons and neutrons are composed of smaller particles yet, called quarks. There are six types of quarks. Two of the most common types are the up quark (u) and the down quark (d). Protons are composed of two up quarks and one down quark (uud), and neutrons are composed of one up quark and two down quarks (ddu), as shown in Figure 1.28. There are hundreds of particles that do not make up chemical elements that can be built from other combinations of two or three quarks.
Each fundamental particle also has a corresponding antimatter particle or antiparticle. Antiparticles are similar to particles except that their charges are reversed. So, for example, an anti-proton has the same mass as a proton, but it has a negative electric charge instead of a positive one. Further, the antiproton would be composed of corresponding groups of anti-quarks, so two anti-up quarks and one anti-down quark. A corresponding scheme would make up the anti-neutron.

Unlike protons and neutrons, which are made up of smaller particles, the electron is itself an elementary particle. Electrons are part of a class of particles known as leptons. As with quarks, there are six types of leptons (and six corresponding anti-leptons). Neutrinos are another example of leptons. Neutrinos have such a small mass and interact so weakly with matter that even though billions of them pass through your body every day - as many coming up from the ground after passing entirely through the Earth as coming down from the sky before then passing on through you and the Earth - that you will never notice them.
The third and final class of fundamental particles consists of the field bosons, which transmit the four fundamental forces of nature. These four forces are: the electromagnetic force, which keeps electrons bound to the nucleus and thereby determines the structure of atoms; the strong nuclear force, which holds the nucleus and the particles in it together; the weak nuclear force, which is important in radioactive decay and other interactions; and gravity, which dominates on astronomical scales. For example, the particle associated with the electromagnetic force is the photon, or particle of light. For the strong nuclear force, an exchange of gluons holds quarks together to form larger particles. If all this sounds very complicated, it sort of is. But don’t worry. We will return to these concepts again in later chapters when we discuss the conditions that were present at the beginning of the Universe. Hopefully with further study this will all become less confusing.
The three classes of elementary particles: quarks, leptons, and field bosons, are summarized in Figure 1.29.
Going larger, molecules are structures that contain two or more atoms bound together by sharing electrons between them. This sharing is called a chemical bond. The atoms of any particular molecule have a definite pattern or structural arrangement that is determined by these bonds. Molecules can have all the same atom or a combination of different atoms. For example, oxygen (O2) is composed of two oxygen atoms, and water (H2O) is made of two hydrogen atoms and one oxygen atom. The size of a molecule is dependent upon the number of bound atoms and the distance between these atoms. Most molecules are so small they are measured in units such as nanometers or micrometers, but sizes can range anywhere from 10-10 m for molecular hydrogen, the smallest molecule, up to 5 cm in the case of a human DNA strand (if it were uncurled). DNA is one example of a rather large molecule that is present in cells, the building blocks of life. There are about 1014 cells in the human body: a typical human cell size is 10-5 m.
| OBJECT | SIZE |
|---|---|
| Quarks | < 10⁻18 m |
| Leptons (including electrons) | < 10⁻18 m |
| Protons | 10⁻15 m |
| Neutrons | 10⁻15 m |
| Atomic nucleus | 10⁻15 to 10⁻14 m |
| Atom | 10⁻10 m |
| Molecule | Typical ~10⁻6 m, range 10⁻10 m – 0.05 m |
| Cell | Typical 10⁻5 m |


