14.2: Temperature Change and Heat Capacity
- Page ID
- 1587
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
By the end of this section, you will be able to:
- Observe heat transfer and change in temperature and mass.
- Calculate final temperature after heat transfer between two objects.
One of the major effects of heat transfer is temperature change: heating increases the temperature while cooling decreases it. We assume that there is no phase change and that no work is done on or by the system. Experiments show that the transferred heat depends on three factors—the change in temperature, the mass of the system, and the substance and phase of the substance.
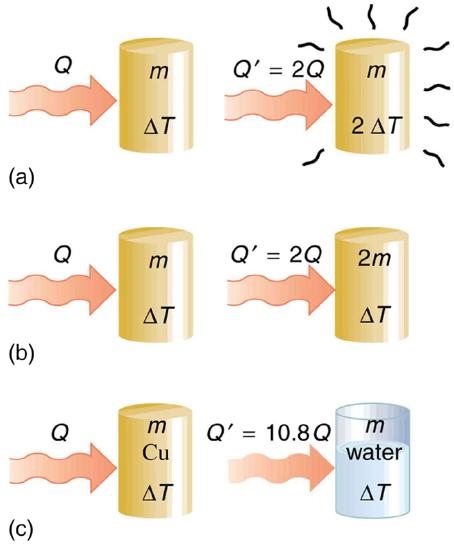
The dependence on temperature change and mass are easily understood. Owing to the fact that the (average) kinetic energy of an atom or molecule is proportional to the absolute temperature, the internal energy of a system is proportional to the absolute temperature and the number of atoms or molecules. Owing to the fact that the transferred heat is equal to the change in the internal energy, the heat is proportional to the mass of the substance and the temperature change. The transferred heat also depends on the substance so that, for example, the heat necessary to raise the temperature is less for alcohol than for water. For the same substance, the transferred heat also depends on the phase (gas, liquid, or solid).
Heat Transfer and Temperature Change
The quantitative relationship between heat transfer and temperature change contains all three factors: \[Q = mc\Delta T,\] where \(Q\) is the symbol for heat transfer, \(m\) is the mass of the substance, and \(\Delta T\) is the change in temperature. The symbol \(c\) stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by \(1.00^oC\). The specific heat \(c\) is a property of the substance; its SI unit is \(J/(kg \cdot K)\) or \(J/(kg \cdot ^oC)\). Recall that the temperature change \((\Delta T)\) is the same in units of kelvin and degrees Celsius. If heat transfer is measured in kilocalories, then the unit of specific heat is \(kcal/(kg \cdot ^oC)\).
Values of specific heat must generally be looked up in tables, because there is no simple way to calculate them. In general, the specific heat also depends on the temperature. Table \(\PageIndex{1}\) lists representative values of specific heat for various substances. Except for gases, the temperature and volume dependence of the specific heat of most substances is weak. We see from this table that the specific heat of water is five times that of glass and ten times that of iron, which means that it takes five times as much heat to raise the temperature of water the same amount as for glass and ten times as much heat to raise the temperature of water as for iron. In fact, water has one of the largest specific heats of any material, which is important for sustaining life on Earth.
Example \(\PageIndex{1}\): Calculating the Required Heat: Heating Water in an Aluminum Pan
A 0.500 kg aluminum pan on a stove is used to heat 0.250 liters of water from \(20.0^oC\) to \(80.0^oC\).
(a) How much heat is required? What percentage of the heat is used to raise the temperature of (b) the pan and (c) the water?
Strategy
The pan and the water are always at the same temperature. When you put the pan on the stove, the temperature of the water and the pan is
increased by the same amount. We use the equation for the heat transfer for the given temperature change and mass of water and aluminum. The specific heat values for water and aluminum are given in Table \(\PageIndex{1}\).
Solution
Because water is in thermal contact with the aluminum, the pan and the water are at the same temperature.
- Calculate the temperature difference: \[\Delta T = T_f - T_i = 60.0^oC.\]
- Calculate the mass of water. Because the density of water is \(1000 \, kg/m^3\), one liter of water has a mass of 1 kg, and the mass of 0.250 liters of water is \(m_w = 0.250 \, kg\).
- Calculate the heat transferred to the water. Use the specific heat of water in Table \(\PageIndex{1}\) \[Q_w = m_wc_w\Delta T = (0.250 \, kg)(4186 \, J/kg ^oC)(60.0^oC) = 62.8 \, kJ.\]
- Calculate the heat transferred to the aluminum. Use the specific heat for aluminum in Table \(\PageIndex{1}\: \[Q_{Al} = m_{Al}c_{Al}\Delta T = (0.500 \, kg)(900 \, J/kg^oC)(60.0^oC) = 27.0 kJ.\]
- Compare the percentage of heat going into the pan versus that going into the water. First, find the total transferred heat: \[Q_{Total} = Q_W + Q_{Al} = 62.8 \, kJ + 27.0 \, kJ = 89.8 \, kJ.\]
- Thus, the amount of heat going into heating the pan is \[\dfrac{62.8 \, kJ}{89.8 \, kJ} \times 100\% = 69.9\%\]
Discussion
In this example, the heat transferred to the container is a significant fraction of the total transferred heat. Although the mass of the pan is twice that of the water, the specific heat of water is over four times greater than that of aluminum. Therefore, it takes a bit more than twice the heat to achieve the given temperature change for the water as compared to the aluminum pan.
Example \(\PageIndex{2}\): Calculating the Temperature Increase from the Work Done on a Substance: Truck Brakes Overheat on Downhill Runs
Truck brakes used to control speed on a downhill run do work, converting gravitational potential energy into increased internal energy (higher temperature) of the brake material. This conversion prevents the gravitational potential energy from being converted into kinetic energy of the truck. The problem is that the mass of the truck is large compared with that of the brake material absorbing the energy, and the temperature increase may occur too fast for sufficient heat to transfer from the brakes to the environment.
Calculate the temperature increase of 100 kg of brake material with an average specific heat of \(800.0 \, J/kg \cdot ^oC\) if the material retains 10% of the energy from a 10,000-kg truck descending 75.0 m (in vertical displacement) at a constant speed.
Strategy
If the brakes are not applied, gravitational potential energy is converted into kinetic energy. When brakes are applied, gravitational potential energy is converted into internal energy of the brake material. We first calculate the gravitational potential energy \((Mgh)\) that the entire truck loses in its descent and then find the temperature increase produced in the brake material alone.
Solution
- Calculate the change in gravitational potential energy as the truck goes downhill \[Mgh = (10,000 \, kg)(9.80 \, m/s^2)(75.0 \, m) = 7.35 \times 10^6 \, J.\]
- Calculate the temperature from the heat transferred using \(Q = Mgh\) and \[\Delta T = \dfrac{Q}{mc},\] where \(m\) is the mass of the brake material. Insert the values \(m = 100 \, kg\) and \(c = 800 \, J/kg \cdot ^oC\) to find \[\Delta T = \dfrac{(7.35v \times 10^6 \, J)}{(100 \, kg)(800 \, J/kg^oC)} = 92^oC.\]
Discussion
This temperature is close to the boiling point of water. If the truck had been traveling for some time, then just before the descent, the brake temperature would likely be higher than the ambient temperature. The temperature increase in the descent would likely raise the temperature of the brake material above the boiling point of water, so this technique is not practical. However, the same idea underlies the recent hybrid technology of cars, where mechanical energy (gravitational potential energy) is converted by the brakes into electrical energy (battery).
| Substances | Specific heat (c) | |
|---|---|---|
| Solids | \(J/kg\cdot^oC\) | \(kcal/kg\cdot^oC\) |
| Aluminum | 900 | 0.215 |
| Asbestos | 800 | 0.19 |
| Concrete, granite (average) | 840 | 0.20 |
| Copper | 387 | 0.0924 |
| Glass | 840 | 0.20 |
| Gold | 129 | 0.0308 |
| Human body (average at 37 °C) | 3500 | 0.83 |
| Ice (average, -50°C to 0°C) | 2090 | 0.50 |
| Iron, steel | 452 | 0.108 |
| Lead | 128 | 0.0305 |
| Silver | 235 | 0.0562 |
| Wood | 1700 | 0.4 |
| Liquids | ||
| Benzene | 1740 | 0.415 |
| Ethanol | 2450 | 0.586 |
| Glycerin | 2410 | 0.576 |
| Mercury | 139 | 0.0333 |
| Water (15.0 °C) | 4186 | 1.000 |
| Gases 3 | ||
| Air (dry) | 721 (1015) | 0.172 (0.242) |
| Ammonia | 1670 (2190) | 0.399 (0.523) |
| Carbon dioxide | 638 (833) | 0.152 (0.199) |
| Nitrogen | 739 (1040) | 0.177 (0.248) |
| Oxygen | 651 (913) | 0.156 (0.218) |
| Steam (100°C) | 1520 (2020) | 0.363 (0.482) |
Note that Example \(\PageIndex{2}\) is an illustration of the mechanical equivalent of heat. Alternatively, the temperature increase could be produced by a blow torch instead of mechanically.
Example \(\PageIndex{3}\): Calculating the Final Temperature When Heat Is Transferred Between Two Bodies: Pouring Cold Water in a Hot Pan
Suppose you pour 0.250 kg of \(20.0^oC\) water (about a cup) into a 0.500-kg aluminum pan off the stove with a temperature of \(150^oC\). Assume that the pan is placed on an insulated pad and that a negligible amount of water boils off. What is the temperature when the water and pan reach thermal equilibrium a short time later?
Strategy
The pan is placed on an insulated pad so that little heat transfer occurs with the surroundings. Originally the pan and water are not in thermal equilibrium: the pan is at a higher temperature than the water. Heat transfer then restores thermal equilibrium once the water and pan are in contact. Because heat transfer between the pan and water takes place rapidly, the mass of evaporated water is negligible and the magnitude of the heat lost by the pan is equal to the heat gained by the water. The exchange of heat stops once a thermal equilibrium between the pan and the water is achieved. The heat exchange can be written as \(|Q_{hot}| = Q_{cold}.\)
Solution
- Use the equation for heat transfer \(Q = mc\Delta T\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan, and the final temperature: \[Q_{hot} = m_{Al}c_{Al}(T_f - 150^oC).\]
- Express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water and the final temperature: \[Q_{cold} = m_wc_w(T_f - 20.0^oC).\]
- Note that \(Q_{hot}<0\) and \(Q_{cold} >0\) and that they must sum to zero because the heat lost by the hot pan must be the same as the heat gained by the cold water: \[Q_{cold} + Q_{hot} = 0,\] \[Q_{cold} = -Q_{hot},\] \[m_wc_w(T_f - 20.0^oC) = -m_{Al}c_{Al}(T_f - 150.0^oC).\]
- Bring all terms involving \(T_f\) on the left hand side and all other terms on the right hand side. Solve for \(T_f\),
\(T_f=\dfrac{m_{Al}c_{Al}(150ºC)+m_Wc_W(20.0ºC)}{m_{Al}c_{Al}+m_Wc_W}\),
and insert the numerical values:
\(T_f=\dfrac{(0.500 kg)(900 J/kgºC)(150ºC)+(0.250 kg)(4186 J/kgºC)(20.0ºC)}{(0.500 kg)(900 J/kgºC)+(0.250 kg)(4186 J/kgºC)}=\dfrac{88430 J}{1496.5 J/ºC}=59.1ºC.\)
Discussion
This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. Why is the final temperature so much closer to 20.0ºC than 150ºC? The reason is that water has a greater specific heat than most common substances and thus undergoes a small temperature change for a given heat transfer. A large body of water, such as a lake, requires a large amount of heat to increase its temperature appreciably. This explains why the temperature of a lake stays relatively constant during a day even when the temperature change of the air is large. However, the water temperature does change over longer times (e.g., summer to winter).
TAKE-HOME EXPERIMENT: TEMPERATURE CHANGE OF LAND AND WATER
What heats faster, land or water?
To study differences in heat capacity:
- Place equal masses of dry sand (or soil) and water at the same temperature into two small jars. (The average density of soil or sand is about 1.6 times that of water, so you can achieve approximately equal masses by using \(50%\) more water by volume.)
- Heat both (using an oven or a heat lamp) for the same amount of time.
- Record the final temperature of the two masses.
- Now bring both jars to the same temperature by heating for a longer period of time.
- Remove the jars from the heat source and measure their temperature every 5 minutes for about 30 minutes.
Which sample cools off the fastest? This activity replicates the phenomena responsible for land breezes and sea breezes.
Exercise \(\PageIndex{1}\)
If 25 kJ is necessary to raise the temperature of a block from 25ºC to 30ºC, how much heat is necessary to heat the block from 45ºC to 50ºC?
- Answer
-
The heat transfer depends only on the temperature difference. Since the temperature differences are the same in both cases, the same 25 kJ is necessary in the second case.
Summary
- The transfer of heat \(Q\) that leads to a change \(ΔT\) in the temperature of a body with mass \(m\) is \(Q=mcΔT\), where \(c\) is the specific heat of the material. This relationship can also be considered as the definition of specific heat.
Footnotes
1 The values for solids and liquids are at constant volume and at 25ºC, except as noted.
2 These values are identical in units of cal/g⋅ºC.
3 cv at constant volume and at 20.0ºC, except as noted, and at 1.00 atm average pressure. Values in parentheses are \(c_p\) at a constant pressure of 1.00 atm.
Glossary
specific heat
the amount of heat necessary to change the temperature of 1.00 kg of a substance by 1.00 ºC


