3.1: Carnot Cycle
( \newcommand{\kernel}{\mathrm{null}\,}\)
A thermodynamic engine operates by taking in heat from a hot reservoir and performing certain work and then giving up a certain amount of heat into a colder reservoir. If it can be operated in reverse, it can function as a refrigerator. The Carnot cycle is a reversible cyclic process (or engine) made of the following four steps:
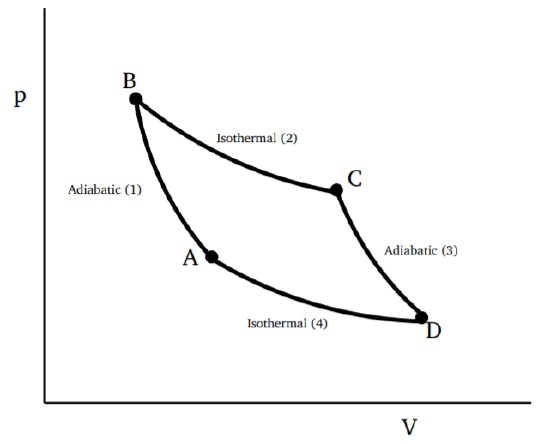
- It starts with an adiabatic process which raises the temperature of the working material of the engine to, say, TH
- This is followed by a isothermal process, taking in heat from the reservoir at TH.
- The next step is an adiabatic process which does some amount of work and lowers the temperature of the material to TL.
- The final step is isothermal, at the lower temperature TL, dumping some amount of heat into a colder reservoir, with the material returning to the thermodynamic state at the beginning of the cycle.
This is an idealized engine, no real engine can be perfectly reversible. The utility of the Carnot engine is to give the framework and logic of the arguments related to the second law of thermodynamics. We may say it is a gedanken engine. The processes involved in the Carnot cycle may refer to compression and expansion if the material is a gas; in this case, the cycle can be illustrated in a p−V diagram as shown in Fig. 3.1.1. But any other pair of thermodynamic variables will do as well. We can think of a Carnot cycle utilizing magnetization and magnetic field, or surface tension and area, or one could consider an electrochemical cell.
Let the amount of heat taken in at temperature TH be QH and let the amount of heat given up at the lower temperature TL be QL. Since this is an idealized case, we assume there is no loss of heat due to anything like friction. Thus the amount of work done, according to the first law is
W=QH−QL
The efficiency of the engine is given by the amount of work done when a given amount of heat is supplied, which is WQH. (The heat QL which is dumped into the reservoir at lower temperature is not usable for work.) The efficiency η for a Carnot cycle is thus
η=QH−QLQH=1−QLQH
The importance of the Carnot cycle is due to its idealized nature of having no losses and because it is reversible. This immediately leads to some simple but profound consequences.


