9.2: Carathéodory Statement of the Second Law
( \newcommand{\kernel}{\mathrm{null}\,}\)
The statement of the second law due to Carathéodory is:
In the neighborhood of any equilibrium state of a physical system with any number of thermodynamic coordinates, there exist states which are inaccessible by adiabatic processes.
The adiabatic processes can be quite general, not necessarily quasi-static. It is easy to see that this leads immediately to the notion of absolute temperature and entropy. This has been discussed in a concise and elegant manner in Chandrasekhar’s book on stellar structure. We briefly repeat his argument for completeness. For simplicity, consider a gas characterized by pressure p and volume V, and (empirical) temperature t, only two of which are adequate to specify the thermodynamic state, the third being given by an equation of state. Since these are the only variables, dQ has an integrating factor and we may write
dQ=τdσ
where σ and τ will be functions of the variables p,V,t. The power of Carathéodory’s formulation becomes clear when we consider two such systems brought into thermal contact and come to equilibrium. We then have a common temperature t and the thermodynamic variables can now be taken as V1,V2,t (or t and one variable from each of (p1,V1),(p2,V2)). We also have dQ=dQ1+dQ2. The number of variables is now three; nevertheless, the Carathéodory principle tells us that we can write
τdσ=τ1dσ1+τ2dσ2
We now choose t,σ1,σ2 as the independent variables. Equation ??? then leads to
∂σ∂σ1=τ1τ,∂σ∂σ2=τ2τ,frac∂σ∂t=0
The last of these equations tells us that σ is only a function of σ1 and σ2, σ=σ(σ1,σ2). Further, since σ is a well-defined function of the various variables, derivatives on σ commute and so
∂∂t∂σ∂σ1−∂σ∂σ1∂σ∂t=0
with a similar relation for derivatives with respect to σ2 as well. Thus we have the result
∂∂t(τ1τ)=0,∂∂t(τ2τ)=0
Equivalently, we can write
1τ1∂τ1∂t=1τ2∂τ2∂t=1τ∂τ∂t
This shows that the combination (1τ)(∂τ∂t) is independent of the system and is a universal function of the common variable t. Taking this function as g(t) and integrating, we get
τ=Σ(σ1,σ2)C exp(∫tt0dtg(t))τ1=Σ1(σ1)C exp(∫tt0dtg(t))τ2=Σ2(σ2)C exp(∫tt0dtg(t))
The τ’s are determined up to a function of the σ’s; we take this arbitrariness as CΣ, where C is a constant and Σ is a function of the σ’s involved. We can now define the absolute temperature as
T≡C exp(∫tt0dtg(t))
Notice that, in the case under consideration, T1=T2=T as expected for equilibrium. This gives dQ1=TΣ1dσ1, etc. The relation dQ=dQ1+dQ2 now reduces to
Σdσ=Σ1dσ1+Σ2dσ2
In the two-dimensional space with coordinates σ1, σ2, the vector (Σ1,Σ2) has vanishing curl, i.e., ∂1Σ2−∂2Σ1=0, since Σ1 only depends on σ1 and similarly for Σ2. Thus Equation ??? shows that Σdσ is a perfect differential. This means that there exists a function S such that Σdσ=dS; this also means that Σ can depend on σ1 and σ2 only through the combination σ(σ1,σ2). Thus finally we have
dQ=TdS
In this way, the Carathéodory principle leads to the definition of entropy S.
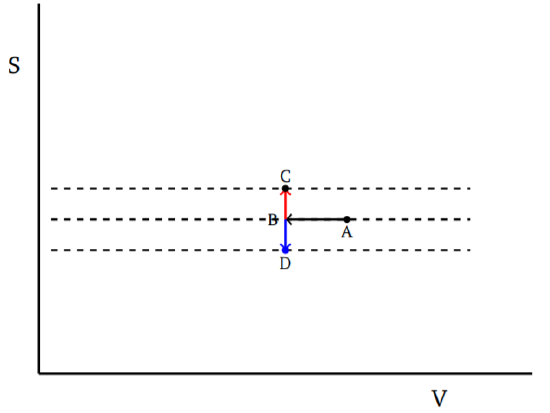
One can also see how this leads to the principle of increase of entropy. For this, consider a system with n thermodynamic variables. The entropy will be a function of these. We can alternatively choose n−1 of the given variables and the entropy S to characterize states of the system. Now we ask the question: Given a state A, can we find a path which takes us via adiabatic processes to another state C? It is useful to visualize this in a diagram, with S as one of the axes, as in Fig. 9.2.1. We show one of the other axes, but there could be many. To get to C, we can start from A and go along a quasi-static reversible adiabatic to B and then, via some nonquasi-static process such as stirring, mixing, etc., get to C, keeping the system in adiabatic isolation. This second process can be irreversible. The idea is that the first part does not change the entropy, but brings the other variables to their desired final value. Then we move to the required value of S by some irreversible process. As shown SC>SB=SA. Suppose the second process can also decrease the entropy in some cases so that we can go from B to D by some similar process. Then we see that all states close to B are accessible. Starting from any point, we can move along the surface of constant S to get to the desired value of the variables, except for S and then jump to the required value of S by the second process. This contradicts the Carathéodory principle. Thus, if we postulate this principle, then we have to conclude that in all irreversible processes in adiabatic isolation the entropy has to either decrease or increase; we cannot have it increase in some processes and decrease in some other processes. So S should be either a nondecreasing quantity or a nonincreasing quantity. The choice of the sign of the absolute temperature, via the choice of the sign of the constant C in Equation ???, is related to which case we choose for entropy. The conventional choice, of course, is to take T≥0 and entropy to be nondecreasing. In other words
∆S ≥ 0
Thus effectively, we have obtained the version of the second law as given in Proposition 4 in Chapter 3.


