30.2: Radioactivity
- Page ID
- 16243
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning objectives
- Name major sources of terrestrial radiation.
Radioactive material is found throughout nature. Detectable amounts occur naturally in soil, rocks, water, air, and vegetation. From these sources it can be inhaled and ingested into the body. In addition to this internal exposure, humans also receive external exposure from radioactive materials that remain outside the body and from cosmic radiation from space. The worldwide average natural dose to humans is about 2.4 millisieverts (mSv) per year. This is four times more than the worldwide average artificial radiation exposure, which in the year 2008 amounted to about 0.6 mSv per year. In some wealthier countries, such as the US and Japan, artificial exposure is, on average, greater than the natural exposure, due to greater access to medical imaging. In Europe, the average natural background exposure by country ranges from under 2 mSv annually in the United Kingdom to more than 7 mSv annually in Finland, as shown in.
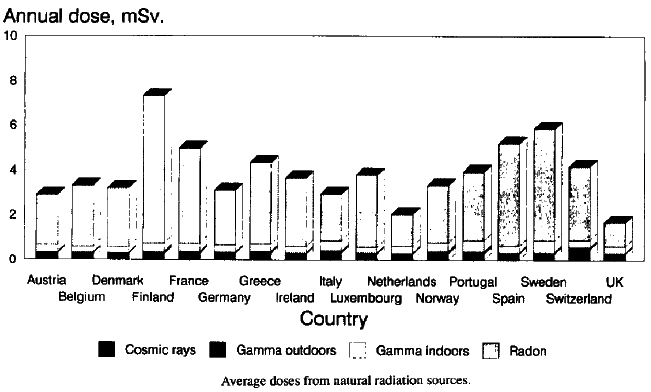
Natural Radiation Atlas of Europe: Bar chart of average annual dosages from natural radiation sources for major European countries
Natural Background Radiation
The biggest source of natural background radiation is airborne radon, a radioactive gas that emanates from the ground. Radon and its isotopes, parent radionuclides, and decay products all contribute to an average inhaled dose of 1.26 mSv/a. Radon is unevenly distributed and variable with weather, such that much higher doses occur in certain areas of the world. In these areas it can represent a significant health hazard. Concentrations over 500 times higher than the world average have been found inside buildings in Scandinavia, the United States, Iran, and the Czech Republic. Radon is a decay product of uranium, which is relatively common in the Earth’s crust but more concentrated in ore-bearing rocks scattered around the world. Radon seeps out of these ores into the atmosphere or into ground water; it can also infiltrate into buildings. It can be inhaled into the lungs, along with its decay products, where it will reside for a period of time after exposure.
Radiation from Outer Space
In addition, the earth, and all living things on it, are constantly bombarded by radiation from outer space. This radiation primarily consists of positively charged ions ranging from protons to iron and larger nuclei derived from sources outside of our solar system. This radiation interacts with atoms in the atmosphere to create an air shower of secondary radiation, including x-rays, muons, protons, alpha particles, pions, electrons, and neutrons. The immediate dose from cosmic radiation is largely from muons, neutrons, and electrons, and this dose varies in different parts of the world based on the geomagnetic field and altitude. This radiation is much more intense in the upper troposphere (around 10 km in altitude) and is therefore of particular concern for airline crews and frequent passengers, who spend many hours per year in this environment. An airline crew typically gets an extra dose on the order of 2.2 mSv (220 mrem) per year.
Terrestrial Radiation
Terrestrial radiation only includes sources that remain external to the body. The major radionuclides of concern are potassium, uranium, and thorium and their decay products. Some of these decay products, like radium and radon, are intensely radioactive but occur in low concentrations. Most of these sources have been decreasing, due to radioactive decay since the formation of the earth, because there is no significant source of replacement. Because of this, the present activity on Earth from uranium-238 is only half as much as it originally was because of its 4.5-billion-year half-life. Potassium-40 (with a half-life of 1.25 billion years) is at about eight percent of its original activity. However, the effects on humans of the actual diminishment (due to decay) of these isotopes is minimal. This is because humans evolved too recently for the difference in activity over a fraction of a half-life to be significant. Put another way, human history is so short in comparison to a half-life of a billion years that the activity of these long-lived isotopes has been effectively constant throughout our time on this planet.
Many shorter-half-life and therefore more intensely radioactive isotopes have not decayed out of the terrestrial environment because they are still being produced. Examples of these are radium-226 (a decay product of uranium-238) and radon-222 (a decay product of radium-226).
Radiation Detection
A radiation detector is a device used to detect, track, or identify high-energy particles.
Learning objectives
- Explain difference between major types of radiation detectors.
A radiation detector is a device used to detect, track, or identify high- energy particles, such as those produced by nuclear decay, cosmic radiation, and reactions in a particle accelerator. Modern detectors are also used as calorimeters to measure the energy of detected radiation. They may be also used to measure other attributes, such as momentum, spin, and charge of the particles. Different types of radiation detectors exist; gaseous ionization detectors, semiconductor detectors, and scintillation detectors are the most common.
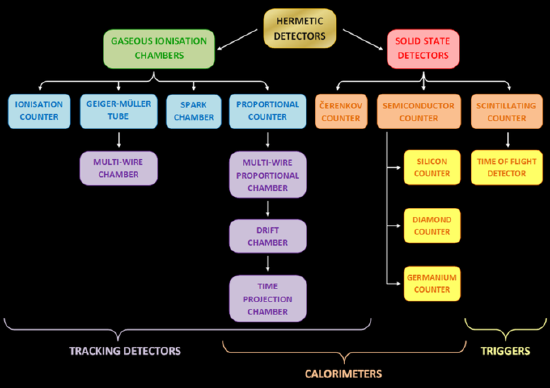
Different Types of Radiation Detectors: different types of radiation detectors (counters)
Gaseous Ionization Detectors
Gaseous ionization detectors use the ionizing effect of radiation upon gas-filled sensors. If a particle has enough energy to ionize a gas atom or molecule, the resulting electrons and ions cause a current flow, which can be measured.
Semiconductor Detectors
A semiconductor detector uses a semiconductor (usually silicon or germanium) to detect traversing charged particles or the absorption of photons. When these detectors’ sensitive structures are based on single diodes, they are called semiconductor diode detectors. When they contain many diodes with different functions, the more general term “semiconductor detector” is used. Semiconductor detectors have had various applications in recent decades, in particular in gamma and x-ray spectrometry and as particle detectors.
Scintillation Detectors
A scintillation detector is created by coupling a scintillator — a material that exhibits luminescence when excited by ionizing radiation — to an electronic light sensor, such as a photomultiplier tube (PMT) or a photodiode. PMTs absorb the light emitted by the scintillator and re-emit it in the form of electrons via the photoelectric effect. The subsequent multiplication of those electrons (sometimes called photo-electrons) results in an electrical pulse, which can then be analyzed. The pulse yields meaningful information about the particle that originally struck the scintillator.
Scintillators are used by the American government, particularly Homeland Security, as radiation detectors. Scintillators can also be used in neutron and high-energy particle physics experiments, new energy resource exploration, x-ray security, nuclear cameras, computed tomography, and gas exploration. Other applications of scintillators include CT scanners and gamma cameras in medical diagnostics, screens in computer monitors, and television sets.
Radioactive Decay Series: Introduction
Radioactive decay series describe the decay of different discrete radioactive decay products as a chained series of transformations.
Learning objectives
- Describe importance of radioactive decay series for decay process.
Radioactive decay series, or decay chains, describe the radioactive decay of different discrete radioactive decay products as a chained series of transformations. Most radioactive elements do not decay directly to a stable state; rather, they undergo a series of decays until eventually a stable isotope is reached.
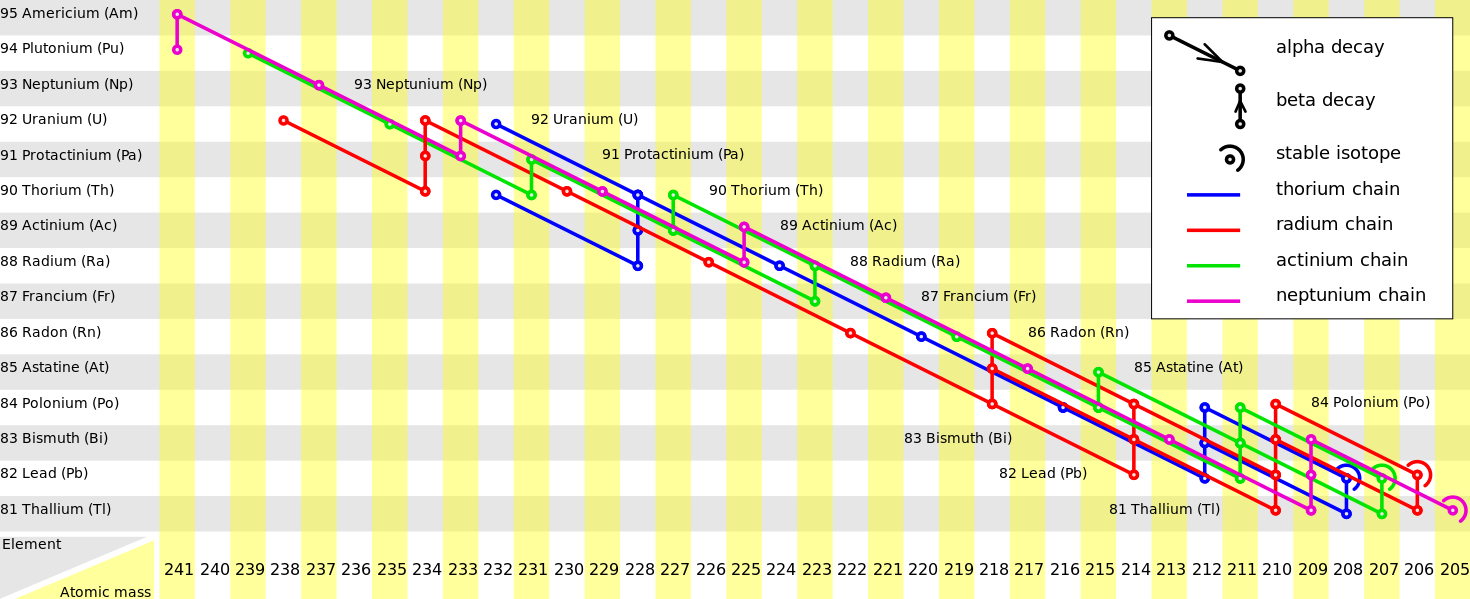
Radioactive Decay Series Diagram: This diagram provides examples of four decay series: thorium (in blue), radium (in red), actinium (in green), and neptunium (in purple).
Decay stages are referred to by their relationship to previous or subsequent stages. A parent isotope is one that undergoes decay to form a daughter isotope. The daughter isotope may be stable, or it may itself decay to form a daughter isotope of its own. The daughter of a daughter isotope is sometimes called a granddaughter isotope.
The time it takes for a single parent atom to decay to an atom of its daughter isotope can vary widely, not only for different parent-daughter chains, but also for identical pairings of parent and daughter isotopes. While the decay of a single atom occurs spontaneously, the decay of an initial population of identical atoms over time, tt, follows a decaying exponential distribution, e−te−t, where λλis called the decay constant. Because of this exponential nature, one of the properties of an isotope is its half-life, the time by which half of an initial number of identical parent radioisotopes have decayed to their daughters. Half-lives have been determined in laboratories for thousands of radioisotopes (radionuclides). These half-lives can range from nearly nonexistent spans of time to as much as \(\mathrm{10^{19}}\) years or more.
The intermediate stages often emit more radioactivity than the original radioisotope. When equilibrium is achieved, a granddaughter isotope is present in proportion to its half-life. But, since its activity is inversely proportional to its half-life, any nuclide in the decay chain finally contributes as much as the head of the chain. For example, natural uranium is not significantly radioactive, but pitchblende, a uranium ore, is 13 times more radioactive because of the radium and other daughter isotopes it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon, a heavy, inert, naturally occurring radioactive gas. Rock containing thorium and/or uranium (such as some granites) emits radon gas, which can accumulate in enclosed places such as basements or underground mines. Radon exposure is considered the leading cause of lung cancer in non-smokers.
Alpha Decay
In alpha decay an atomic nucleus emits an alpha particle and transforms into an atom with smaller mass (by four) and atomic number (by two).
Learning objectives
- Describe the process, penetration power, and effects of alpha radiation
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle that consists of two protons and two neutrons, as shown in. As the result of this process, the parent atom transforms (“decays”) into a new atom with a mass number smaller by four and an atomic number smaller by two.
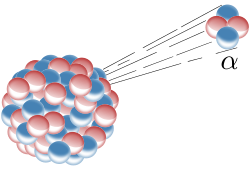
Alpha Decay: Alpha decay is one type of radioactive decay. An atomic nucleus emits an alpha particle and thereby transforms (“decays”) into an atom with a mass number smaller by four and an atomic number smaller by two. Many other types of decay are possible.
For example: \(\mathrm{238U → 234Th + α}\)
Because an alpha particle is the same as a helium-4 nucleus, which has mass number 4 and atomic number 2, this can also be written as:
\(\mathrm{238}\)
\(\mathrm{92U → 234}\)
\(\mathrm{90Th + 4}\)
\(\mathrm{2He}\)
The alpha particle also has charge +2, but the charge is usually not written in nuclear equations, which describe nuclear reactions without considering the electrons. This convention is not meant to imply that the nuclei necessarily occur in neutral atoms.
Alpha decay is by far the most common form of cluster decay, in which the parent atom ejects a defined daughter collection of nucleons, leaving another defined product behind (in nuclear fission, a number of different pairs of daughters of approximately equal size are formed). Alpha decay is the most common cluster decay because of the combined extremely high binding energy and relatively small mass of the helium-4 product nucleus (the alpha particle).
Alpha decay typically occurs in the heaviest nuclides. In theory it can occur only in nuclei somewhat heavier than nickel (element 28), in which overall binding energy per nucleon is no longer a minimum and the nuclides are therefore unstable toward spontaneous fission-type processes. The lightest known alpha emitters are the lightest isotopes (mass numbers 106-110) of tellurium (element 52).
Alpha particles have a typical kinetic energy of 5 MeV (approximately 0.13 percent of their total energy, i.e., 110 TJ/kg) and a speed of 15,000 km/s. This corresponds to a speed of around 0.05 c. There is surprisingly small variation in this energy, due to the heavy dependence of the half-life of this process on the energy produced.
Because of their relatively large mass, +2 electric charge, and relatively low velocity, alpha particles are very likely to interact with other atoms and lose their energy, so their forward motion is effectively stopped within a few centimeters of air.
Most of the helium produced on Earth (approximately 99 percent of it) is the result of the alpha decay of underground deposits of minerals containing uranium or thorium. The helium is brought to the surface as a byproduct of natural gas production.
Beta Decay
Beta decay is a type of radioactive decay in which a beta particle (an electron or a positron) is emitted from an atomic nucleus.
Learning objectives
- Explain difference between beta minus and beta plus decays.
Beta decay is a type of radioactive decay in which a beta particle (an electron or a positron) is emitted from an atomic nucleus, as shown in. Beta decay is a process that allows the atom to obtain the optimal ratio of protons and neutrons.
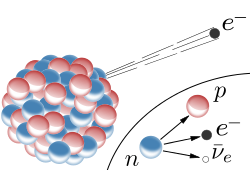
Beta Decay: β decay in an atomic nucleus (the accompanying antineutrino is omitted). The inset shows beta decay of a free neutron
There are two types of beta decay. Beta minus (β) leads to an electron emission (e−); beta plus (β+) leads to a positron emission (e+). In electron emission an electron antineutrino is also emitted, while positron emission is accompanied by an electron neutrino. Beta decay is mediated by the weak force.
Emitted beta particles have a continuous kinetic energy spectrum, ranging from 0 to the maximal available energy (Q), that depends on the parent and daughter nuclear states that participate in the decay. The continuous energy spectra of beta particles occur because Q is shared between a beta particle and a neutrino. A typical Q is around 1 MeV, but it can range from a few keV to a several tens of MeV. Since the rest mass energy of the electron is 511 keV, the most energetic beta particles are ultrarelativistic, with speeds very close to the speed of light.
Since the proton and neutron are part of an atomic nucleus, beta decay processes result in transmutation of one chemical element into another. For example:
\[\mathrm{137Cs → 137Ba + e^-}\]
\[\mathrm{11Na → 10Ne + e^+}\]
Beta decay does not change the number of nucleons, A, in the nucleus; it changes only its charge, Z. Therefore the set of all nuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay.
A beta-stable nucleus may undergo other kinds of radioactive decay (for example, alpha decay). In nature, most isotopes are beta-stable, but there exist a few exceptions with half-lives so long that they have not had enough time to decay since the moment of their nucleosynthesis. One example is the odd-proton odd-neutron nuclide 40 K, which undergoes both types of beta decay with a half-life of 1.277 ·109 years.
Beta Decay 1/2: In this video I introduce Beta decay and discuss it from an basic level to a perhaps second or third year University level.
Beta Decay 2/2: In this video I introduce Beta decay and discuss it from an basic level to a perhaps second or third year University level.
Gamma Decay
Gamma decay is a process of emission of gamma rays that accompanies other forms of radioactive decay, such as alpha and beta decay.
Learning objectives
- Explain relationship between gamma decay and other forms of nuclear decay.
Gamma radiation, also known as gamma rays and denoted as γγ, is electromagnetic radiation of high frequency and therefore high energy. Gamma rays typically have frequencies above 10 exahertz (>1019>1019 Hz) and therefore have energies above 100 keV and wavelengths less than 10 picometers (less than the diameter of an atom). However, this is not a strict definition; rather, it is a rule-of-thumb description for natural processes. Gamma rays from radioactive decay are defined as gamma rays no matter what their energy, so there is no lower limit to gamma energy derived from radioactive decay. Gamma decay commonly produces energies of a few hundred keV and usually less than 10 MeV.
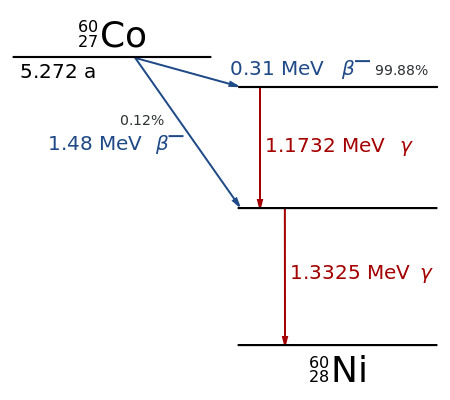
Cobalt-60 Decay Scheme: Path of decay of Co-60 to Ni-60. Excited levels for Ni-60 that drop to ground state via emission of gamma rays are indicated
Gamma decay accompanies other forms of decay, such as alpha and beta decay; gamma rays are produced after the other types of decay occur. When a nucleus emits an α or β particle, the daughter nucleus is usually left in an excited state. It can then move to a lower energy state by emitting a gamma ray, in much the same way that an atomic electron can jump to a lower energy state by emitting a photon. For example, cobalt-60 decays to excited nickel-60 by beta decay through emission of an electron of 0.31 MeV. Next, the excited nickel-60 drops down to the ground state by emitting two gamma rays in succession (1.17 MeV, then 1.33 MeV), as shown in. Emission of a gamma ray from an excited nuclear state typically requires only 10−1210−12 seconds: it is nearly instantaneous. Gamma decay from excited states may also follow nuclear reactions such as neutron capture, nuclear fission, or nuclear fusion.
In certain cases, the excited nuclear state following the emission of a beta particle may be more stable than average; in these cases it is termed a metastable excited state if its decay is 100 to 1000 times longer than the average 10−1210−12 seconds. Such nuclei have half-lives that are easily measurable; these are termed nuclear isomers. Some nuclear isomers are able to stay in their excited state for minutes, hours, or days, or occasionally far longer, before emitting a gamma ray. This phenomenon is called isomeric transition. The process of isomeric transition is therefore similar to any gamma emission; it differs only in that it involves the intermediate metastable excited states of the nuclei.
Half-Life and Rate of Decay; Carbon-14 Dating
Carbon-14 dating is a radiometric dating method that uses the radioisotope carbon-14 (14C) to estimate the age of object.
Learning objectives
- Identify the age of materials that can be approximately determined using radiocarbon dating
Radiocarbon dating (usually referred to simply as carbon-14 dating) is a radiometric dating method. It uses the naturally occurring radioisotope carbon-14 (14C) to estimate the age of carbon-bearing materials up to about 58,000 to 62,000 years old.
Carbon has two stable, nonradioactive isotopes: carbon-12 (12C) and carbon-13 (13C). There are also trace amounts of the unstable radioisotope carbon-14 (14C) on Earth. Carbon-14 has a relatively short half-life of 5,730 years, meaning that the fraction of carbon-14 in a sample is halved over the course of 5,730 years due to radioactive decay to nitrogen-14. The carbon-14 isotope would vanish from Earth’s atmosphere in less than a million years were it not for the constant influx of cosmic rays interacting with molecules of nitrogen (N2) and single nitrogen atoms (N) in the stratosphere. Both processes of formation and decay of carbon-14 are shown in.
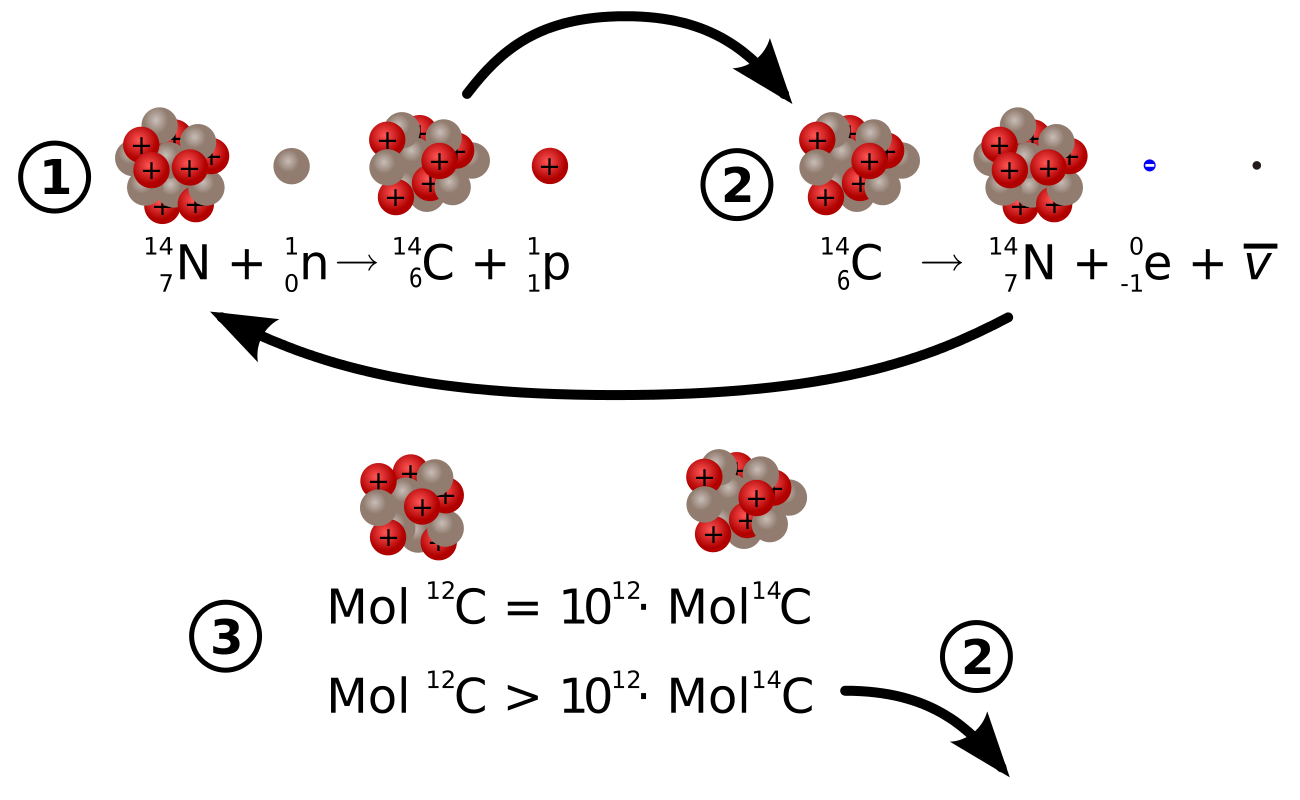
Formation and Decay of Carbon-14: Diagram of the formation of carbon-14 (1), the decay of carbon-14 (2), and equations describing the carbon-12:carbon-14 ratio in living and dead organisms
When plants fix atmospheric carbon dioxide (CO2) into organic compounds during photosynthesis, the resulting fraction of the isotope 14C in the plant tissue will match the fraction of the isotope in the atmosphere. After plants die or are consumed by other organisms, the incorporation of all carbon isotopes, including 14C, stops. Thereafter, the concentration (fraction) of 14C declines at a fixed exponential rate due to the radioactive decay of 14C. (An equation describing this process is shown in. ) Comparing the remaining 14C fraction of a sample to that expected from atmospheric 14C allows us to estimate the age of the sample.
Raw (i.e., uncalibrated) radiocarbon ages are usually reported in radiocarbon years “Before Present” (BP), with “present” defined as CE 1950. Such raw ages can be calibrated to give calendar dates. One of the most frequent uses of radiocarbon dating is to estimate the age of organic remains from archaeological sites.
The technique of radiocarbon dating was developed by Willard Libby and his colleagues at the University of Chicago in 1949. Emilio Segrè asserted in his autobiography that Enrico Fermi suggested the concept to Libby at a seminar in Chicago that year. Libby estimated that the steady-state radioactivity concentration of exchangeable carbon-14 would be about 14 disintegrations per minute (dpm) per gram. In 1960, Libby was awarded the Nobel Prize in chemistry for this work. He demonstrated the accuracy of radiocarbon dating by accurately estimating the age of wood from a series of samples for which the age was known, including an ancient Egyptian royal barge dating from 1850 BCE.
Half-life: Describes radioactive half life and how to do some simple calculations using half life.
Calculations Involving Half-Life and Decay-Rates
The half-life of a radionuclide is the time taken for half the radionuclide’s atoms to decay.
Learning objectives
- Explain what is a half-life of a radionuclide.
The half-life of a radionuclide is the time taken for half of the radionuclide’s atoms to decay. Taking λλ to be the decay rate (number of disintegrations per unit time), and ττ the average lifetime of an atom before it decays, we have:
\[\mathrm { N } ( \mathrm { t } ) = \mathrm { N } _ { 0 } \mathrm { e } ^ { - \lambda \mathrm { t } } = \mathrm { N } _ { 0 } \mathrm { e } ^ { - \mathrm { t } / \tau }\]
The half-life is related to the decay constant by substituting the condition \(\mathrm { N } = \mathrm { N } _ { \mathrm { o } } / 2\) and solving for \(\mathrm{t = t _ { 1 / 2 }}\):
\[\mathrm { t } _ { 1 / 2 } = \ln 2 / \lambda = \tau \ln 2\]
A half-life must not be thought of as the time required for exactly half of the atoms to decay.

Radioactive decay simulation: A simulation of many identical atoms undergoing radioactive decay, starting with four atoms (left) and 400 atoms (right). The number at the top indicates how many half-lives have elapsed
The following figure shows a simulation of many identical atoms undergoing radioactive decay. Note that after one half-life there are not exactly one-half of the atoms remaining; there are only approximatelyone-half left because of the random variation in the process. However, with more atoms (the boxes on the right), the overall decay is smoother and less random-looking than with fewer atoms (the boxes on the left), in accordance with the law of large numbers.
The relationship between the half-life and the decay constant shows that highly radioactive substances are quickly spent while those that radiate weakly endure longer. Half-lives of known radionuclides vary widely, from more than 1019 years, such as for the very nearly stable nuclide 209 Bi, to 10−23 seconds for highly unstable ones.
The factor of ln(2) in the above equations results from the fact that the concept of “half-life” is merely a way of selecting a different base other than the natural base e for the lifetime expression. The time constant τis the e-1-life, the time until only 1/e remains — about 36.8 percent, rather than the 50 percent in the half-life of a radionuclide. Therefore, τis longer than t1/2. The following equation can be shown to be valid:
\[\mathrm { N } ( \mathrm { t } ) = \mathrm { N } _ { 0 } \mathrm { e } ^ { - \mathrm { t } / \tau } = \mathrm { N } _ { 0 } 2 ^ { - \mathrm { t } / \mathrm { t } _ { 1 / 2 } }\]
Since radioactive decay is exponential with a constant probability, each process could just as easily be described with a different constant time period that (for example) gave its 1/3-life (how long until only 1/3 is left), or its 1/10-life (how long until only 1/10 is left), and so on. Therefore, the choice of τand t1/2 for marker-times is only for convenience and for the sake of uploading convention. These marker-times reflect a fundamental principle only in that they show that the same proportion of a given radioactive substance will decay over any time period you choose.
Mathematically, the nth life for the above situation would be found by the same process shown above — by setting \(\mathrm { N } = \mathrm { N } _ { 0 } / \mathrm { n }\) and substituting into the decay solution, to obtain:
\[\mathrm{t _ { 1 / n }} = \dfrac { \ln \mathrm { n } } { \lambda } = \tau \ln \mathrm{n}\]
Half-life: Part of a series of videos on physics problem-solving. The problems are taken from “The Joy of Physics. ” This one deals with radioactive half-life. The viewer is urged to pause the video at the problem statement and work the problem before watching the rest of the video.
Key Points
- The biggest source of natural background radiation is airborne radon, a radioactive gas that emanates from the ground.
- The earth is constantly bombarded by radiation from outer space that consists of positively charged ions ranging from protons to iron and larger nuclei from sources outside of our solar system.
- Terrestrial radiation includes sources that remain external to the body. The major radionuclides of concern are potassium, uranium, and thorium and their decay products.
- Gaseous ionization detectors use the ionizing effect of radiation upon gas-filled sensors.
- A semiconductor detector uses a semiconductor (usually silicon or germanium) to detect traversing charged particles or the absorption of photons.
- A scintillation detector is created by coupling a scintillator to an electronic light sensor.
- Most radioactive elements do not decay directly to a stable state; rather, they undergo a series of decays until eventually a stable isotope is reached.
- Half-lives of radioisotopes range from nearly nonexistent spans of time to as much as 1019 years or more.
- The intermediate stages of radioactive decay series often emit more radioactivity than the original radioisotope.
- An alpha particle is the same as a helium-4 nucleus, which has mass number 4 and atomic number 2.
- Because of their relatively large mass, +2 electric charge, and relatively low velocity, alpha particles are very likely to interact with other atoms and lose their energy, so their forward motion is effectively stopped within a few centimeters of air.
- Most of the helium produced on Earth (approximately 99 percent of it) is the result of the alpha decay of underground deposits of minerals containing uranium or thorium.
- There are two types of beta decay: beta minus, which leads to an electron emission, and beta plus, which leads to a positron emission.
- Beta decay allows the atom to obtain the optimal ratio of protons and neutrons.
- Beta decay processes transmute one chemical element into another.
- Gamma decay accompanies other forms of decay, such as alpha and beta decay; gamma rays are produced after the other types of decay occur.
- Although emission of gamma ray is a nearly instantaneous process, it can involve intermediate metastable excited states of the nuclei.
- Gamma rays are generally the most energetic form of electromagnetic radiation.
- Carbon-14 dating can be used to estimate the age of carbon-bearing materials up to about 58,000 to 62,000 years old.
- The carbon-14 isotope would vanish from Earth’s atmosphere in less than a million years were it not for the constant influx of cosmic rays interacting with atmospheric nitrogen.
- One of the most frequent uses of radiocarbon dating is to estimate the age of organic remains from archaeological sites.
- The half-life is related to the decay constant as follows: \(\mathrm { t } _ { 1 / 2 } = \ln 2 / \lambda\).
- The relationship between the half-life and the decay constant shows that highly radioactive substances are quickly spent while those that radiate weakly endure longer.
- Half-lives of known radionuclides vary widely, from more than 1019 years, such as for the very nearly stable nuclide 209 Bi, to 10-23 seconds for highly unstable ones.
Key Terms
- radionuclide: A radionuclide is an atom with an unstable nucleus, characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or via internal conversion.
- radon: a radioactive chemical element (symbol Rn, formerly Ro) with atomic number 86; one of the noble gases
- sievert: in the International System of Units, the derived unit of radiation dose; the dose received in one hour at a distance of 1 cm from a point source of 1 mg of radium in a 0.5 mm thick platinum enclosure; symbol: Sv
- scintillator: any substance that glows under the action of photons or other high-energy particles
- diode: an electronic device that allows current to flow in one direction only; a valve
- semiconductor: A substance with electrical properties intermediate between a good conductor and a good insulator.
- half-life: the time required for half of the nuclei in a sample of a specific isotope to undergo radioactive decay
- radioisotope: a radioactive isotope of an element
- decay: to change by undergoing fission, by emitting radiation, or by capturing or losing one or more electrons
- alpha particle: A positively charged nucleus of a helium-4 atom (consisting of two protons and two neutrons), emitted as a consequence of radioactivity; α-particle.
- radioactive decay: any of several processes by which unstable nuclei emit subatomic particles and/or ionizing radiation and disintegrate into one or more smaller nuclei
- beta decay: a nuclear reaction in which a beta particle (electron or positron) is emitted
- positron: The antimatter equivalent of an electron, having the same mass but a positive charge.
- transmutation: the transformation of one element into another by a nuclear reaction
- electromagnetic radiation: radiation (quantized as photons) consisting of oscillating electric and magnetic fields oriented perpendicularly to each other, moving through space
- gamma ray: A very high frequency (and therefore very high energy) electromagnetic radiation emitted as a consequence of radioactivity.
- radiometric dating: Radiometric dating is a technique used to date objects based on a comparison between the observed abundance of a naturally occurring radioactive isotope and its decay products using known decay rates.
- carbon-14: carbon-14 is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons.
- radionuclide: A radionuclide is an atom with an unstable nucleus, characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or via internal conversion.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Natural radioactivity. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Natural_radioactivity. License: CC BY-SA: Attribution-ShareAlike
- radon. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radon. License: CC BY-SA: Attribution-ShareAlike
- radionuclide. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/radionuclide. License: CC BY-SA: Attribution-ShareAlike
- sievert. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/sievert. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Gaseous ionization detector. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Gaseous...ation_detector. License: CC BY-SA: Attribution-ShareAlike
- Scintillation detector. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Scintillation_detector. License: CC BY-SA: Attribution-ShareAlike
- Semiconductor detector. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Semiconductor_detector. License: CC BY-SA: Attribution-ShareAlike
- Radiation detector. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radiation_detector. License: CC BY-SA: Attribution-ShareAlike
- scintillator. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/scintillator. License: CC BY-SA: Attribution-ShareAlike
- diode. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/diode. License: CC BY-SA: Attribution-ShareAlike
- semiconductor. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/semiconductor. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._summary_3.png. License: CC BY-SA: Attribution-ShareAlike
- Radioactive decay series. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radioactive_decay_series. License: CC BY-SA: Attribution-ShareAlike
- decay. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/decay. License: CC BY-SA: Attribution-ShareAlike
- half-life. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/half-life. License: CC BY-SA: Attribution-ShareAlike
- radioisotope. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radioisotope. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._summary_3.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...iagram.svg.png. License: CC BY-SA: Attribution-ShareAlike
- alpha particle. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/alpha_particle. License: CC BY-SA: Attribution-ShareAlike
- Alpha decay. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Alpha_decay. License: CC BY-SA: Attribution-ShareAlike
- radioactive decay. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radioactive_decay. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._summary_3.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...iagram.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta decay. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Beta_decay. License: CC BY-SA: Attribution-ShareAlike
- Beta decay. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Beta_decay. License: CC BY-SA: Attribution-ShareAlike
- transmutation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/transmutation. License: CC BY-SA: Attribution-ShareAlike
- positron. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/positron. License: CC BY-SA: Attribution-ShareAlike
- beta decay. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/beta_decay. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._summary_3.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...iagram.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 2/2. Located at: http://www.youtube.com/watch?v=r2Q6xdsGYfE. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 1/2. Located at: http://www.youtube.com/watch?v=4Et47PE288U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Gamma decay. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Gamma_d...ray_production. License: CC BY-SA: Attribution-ShareAlike
- gamma ray. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/gamma_ray. License: CC BY-SA: Attribution-ShareAlike
- electromagnetic radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/electr...etic_radiation. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._summary_3.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...iagram.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 2/2. Located at: http://www.youtube.com/watch?v=r2Q6xdsGYfE. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 1/2. Located at: http://www.youtube.com/watch?v=4Et47PE288U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...Scheme.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Carbon-14. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Carbon-14. License: CC BY-SA: Attribution-ShareAlike
- Radiocarbon dating. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radiocarbon_dating. License: CC BY-SA: Attribution-ShareAlike
- radiometric dating. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/radiometric%20dating. License: CC BY-SA: Attribution-ShareAlike
- radioisotope. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radioisotope. License: CC BY-SA: Attribution-ShareAlike
- carbon-14. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/carbon-14. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._summary_3.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...iagram.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 2/2. Located at: http://www.youtube.com/watch?v=r2Q6xdsGYfE. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 1/2. Located at: http://www.youtube.com/watch?v=4Et47PE288U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...Scheme.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Half-life. Located at: http://www.youtube.com/watch?v=4UPmy_XofMo. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Radioactive decay. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radioac...ay%23Half-life. License: CC BY-SA: Attribution-ShareAlike
- Decay rate. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Decay_rate. License: CC BY-SA: Attribution-ShareAlike
- Half-life. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Half-life. License: CC BY-SA: Attribution-ShareAlike
- half-life. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/half-life. License: CC BY-SA: Attribution-ShareAlike
- radionuclide. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/radionuclide. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._of_Europe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/Wikipedia/commons/thumb/c/c0/Detectors_summary_3.png/800px-Detectors_summary_3.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/Wikipedia/commons/thumb/6/62/Radioactive_decay_chains_diagram.svg/800px-Radioactive_decay_chains_diagram.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/Wikipedia/commons/thumb/7/79/Alpha_Decay.svg/250px-Alpha_Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 2/2. Located at: http://www.youtube.com/watch?v=r2Q6xdsGYfE. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Beta Decay 1/2. Located at: http://www.youtube.com/watch?v=4Et47PE288U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...Scheme.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Half-life. Located at: http://www.youtube.com/watch?v=4UPmy_XofMo. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Half-life. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Half-life. License: Public Domain: No Known Copyright
- Half-life. Located at: http://www.youtube.com/watch?v=zYNpxqkRYlM. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license

