38.2: Introduction
( \newcommand{\kernel}{\mathrm{null}\,}\)
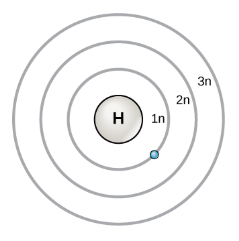
The shell model identifies the number of electrons in each main energy shell of an atom, when the atom is in the ground state. Each element in a vertical group of the periodic table has similar physical and chemical characteristics because all elements in a vertical group have the same electron configurations. The electron configuration also determines the reactivity of an element. By accounting for electrons in the main energy levels (shells), and recognizing that electrons begin to pair-up once a shell is half full, this model can be used to predict chemical bonds.