1.4: Glycolipids
- Page ID
- 1979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Glycolipids are components of cellular membranes comprised of a hydrophobic lipid tail and one or more hydrophilic sugar groups linked by a glycosidic bond. Generally, glycolipids are found on the outer leaflet of cellular membranes where it plays not only a structural role to maintain membrane stability but also facilitates cell-cell communication acting as receptors, anchors for proteins and regulators of signal transduction [1]. Glycolipids are found widely distributed throughout all cells and primarily localized, but not exclusively, to the plasma membrane.
Structure and Synthesis
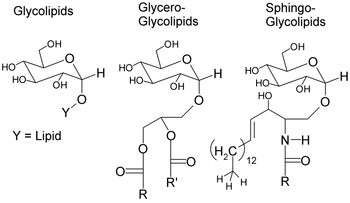
The basic structure of a glycolipid consists of a mono- or oligosaccharide group attached to a sphingolipid or a glycerol group (can be acetylated or alkylated) with one or two fatty acids. These make up the classes of glycosphingolipids and glycoglycerolipids, respectively. Glycolipids interact and bind to the lipid-bilayer through the hydrophobic nature of the lipid tail which anchors it to the surface of the plasma membrane.
Synthesis of glycolipids proceed by a series of enzymes that sequentially add sugars to the lipid. Glycosphingolipids are derived from lactosylceramide (LacCer; β-D-galactosyl(1→4)-β-D-glucosyl-ceramide) where the first step is the acylation and desaturation of D-erythro-sphinganine. Ceramide is glucosylated then β-galactosylated extracellularly to form lactosylceramide. Further elongation can occur via glycosyltransferases and sulfotransferases. For example, the biosynthesis of a major glycoglycerolipid in plants involves the transfer of a galactosyl from UDP-Gal onto diacylglycerol to produce β-galactosyldiacylglycerol via galactosyltransferases. An additional transfer of a galactosyl from UDP-Gal forms α-D-galactosyl-(1,6)-O-β-D-galactosyldiacylglycerol [2].
Metabolism
Degradation of glycosphingolipids are mediated by internalization by endocytosis. They are transported to the lysosomes where enzymes degrade the glycosphingolipids through hydrolytically and irreversible cleavage of bonds. Sphingolipidoses, which are present in the membrane, also mediate the degradation of these class of glycolipids [4].
Dysfunction of glycolipid metabolism is linked to several different diseases from the disruption of glycolipid degradation leading to the accumulation of glycolipids. Figure \(\PageIndex{2}\) illustrates diseases associated with different disruptions in the metabolism of glycolipids. For example, Tay-Sachs disease is an autosomal recessive disease that is a member of the GM2 gangliosidotic disease. The disease portrays symptoms of severe psycho-motor developmental disordered which is caused by the inabiligy to properly degrade membrane associated gangliosides. This occurs because the enzyme required to break down gangliosides, β-hexosamindase A, is dysfunctional due to mutations in the HEXA gene. The accumulation of these gangliosides in the neurons results in neural cell death.
Distribution
The majority of glycolipids are located in membrane structures in the cell. Two-thirds of total glycolipids are distributed in intracellular membranes such as the golgi-apparatus, endosomes, lysosomes, nuclear membrane, and mitochondria [4]. Glycolipids are synthesized in the golgi-apparatus where the majority are transported to membranes to maintain the bilayer. Few glycolipids can be found in the cystol; approximately 5% of the total glycolipids in the brain are found in the soluble fraction.
Glycosphingolipids in the plasma membrane can cluster with cholesterol to for rafts which contains less phospholipids relative to other portions of the membrane; approximately 70% of total glycolipids are found in these rafts which form hydrophobic interactions [5]. In addition, sphingolipids and glycosphingolipids form weak interactions between the carbohydrate head groups and the hydrophobic saturated side chain lipids with cholesterol filling any voids [6]. The strong interactions between glycolipids and cholesterol is the driving force which segregates them from the fluid phospholipids in the membrane [7].
Function
Carbohydrates on glycolipids are the most exposed structures on the extracellular surface of cells and are flexible with numerous binding sites which make them optimal for cell signaling. Since the lipid moiety is usually buried within the membrane, carbohydrate-carbohydrate interactions are the predominant interactions that may occur between glycolipids. They can interact side by side within the same membrane or trans interactions between two membranes. Trans interactions between glycolipids was reported to be the basis for glycosphingolipid-dependent cell to cell adhesion which involves calcium ions [8]. Further studies reported that cell surface carbohydrates play major roles in cell-substrate recognition in oncogenesis, myelin sheath regulation, and cell adhesion in metastasis [9-11]. Glycolipids play an important role in several biological functions such as recognition and cell signaling events; below are a few biological functions glycolipids play a role in.
Signal Transduction
Glycosphingolipids and sphingomyelin are clustured into microdomains where they can associate with serveral different proteins such as cSrc, G-proteins, and focal adhesion kinase to mediate cellular events [12]. In the plasma membrane, glycosphingolipids form rafts with cholesterol where these regions have relatively less phospholipids. Shown in Figure \(\PageIndex{3}\), glycosphingolipids form rafts with cholestorol to anchor GPI proteins to the extracellular leaflet and src family kinases to the cystolic leaflet. Thus, glycosphingolipids have been These microdomains can cause cellular responses by associating with GPI-anchored proteins which may induce activation of specific kinases to transduce the phosphorylation of different substrates [13]. The glycosphingolipid microdomains have also been associated with mediating immunoreceptors and growth factor receptors [14].

Cell Proliferation
Glycolipids have been observed to play a role in the regulation of cell growth via interactions with growth factor receptors. Intracellular ceramide stimulated DNA synthesis in endothelial smooth muscle cells and also induced mitogenesis by platelet-derived growth factor [15]. Lactosylceramide activates NADPH oxidase to modulate interacellular adhesion molecule -1 expression on human umbilical vein endotheial cells and to induce proliferation of human aortic smooth muscle cells. With the reduction of ceramide, there was increased ceramidase activity, sphingomyelin synthase which is associated with the proliferation of smooth muscle cells. In addition, gangliosides are known to be involved in inducing apoptosis. Apoptotic signal triggered by CD95 in lymphoid and myeloid tumor cells increase ceramide levels which results in the increase in ganglioside GD3 synthesis; GD3 is known to be a potent mediatior of cell death. Abundant amounts of glycosphingolipids are found in the plasma membrane of cancer cells where antibodies targeting these gangliosides result in apoptosis [16]. Treatment with anti-ganglioside GD2 monoclonal antibodies induces apoptosis in GD2 expressing human lung cancer cells.
Calcium Signaling
Gangliosides are associated with calcium ions which is thought to have a role in neuronal function. Ganglioslide micelles bind to calcium ions with high affinity and may play a significant role in synaptic transmission. It has been reported that sphingosine and ceramide mediate the release of calcium from intracellular stores. Gangliosides may also play a role in calcium homeostasis and signaling. These glycolipids induce changes in cellular calcium through the modulation ofcalcium influx channels, calcium exchange proteins, and calcium dependent enzymes which were altered through the association of gangliosides. [17]. In addition, increased levels of intracellular glucosylceramide resulted in increased calcium stores in neurons [18]. Glycolipid galactocerebroside have been observed in the opening of calcium channels in oligodendrocyte cells.
Types of Glycolipids
Glycosphingolipids
Glycosphingolipids are a class of glycolipids which contain ceramide as the lipid complex. Ceramides are amides of fatty acids with long chain di- or trihydroxy bases. The acyl group of ceramides is a long chain saturated or monounsaturated fatty acids. These lipids are primarily found in nerve tissue nerve tissue and mediate cell signaling. The glycophingolipids can be subdivided into the following groups:
- Neutral glycosphingolipids: Cerebrosides also known as monoglycosylceramides are glycolipids primarily found in the brain and peripheral nervous tissue. The most common cerebroside contains a molecule of galactose found in myelin. Their function is to provide a protective coating to each nerve acting as an insulator with high concentrations in the myelin sheath.
- Acidic glycosphingolipids: These lipids are negatively charged at physiological pH which is provided by N-acetylneuraminic acid (NANA) or by sulfate groups in sulfatides. Gangliosides contain a sialic acid (NANA) making them negatively charged. These are found in the ganglion cells of the CNS and are predominantly at nerve endings. Sulfatides are sulfated galactocerebrosides which are found in the brain and kidneys. They are primarily found in the medulated nerve fibers and have been linked to immune responses to nervous system signaling.
- Basic glycosphingolipids
- Amphoteric glycosphingolipids
Glycoglycerolipids
- Neutral glycoglycerolipids: These usually contain one or two sugars linked to glycerol or diacylglycerol. These lipids have important roles in higher plants, algae, and bacteria in which they are localized to photosynthetic membranes. Photosynthetic membranes are comprised of about 85% of neutral glycoglycerolipids [6]. neutral glycoglycerol lipids can be separated into non-acylated or acylated glycoside moieties.
- Glycophospholipids: These compounds are glycoglycerolipids containing at least one phosphate group attached to either the sugar or glycerol. The simpliest of these compounds are found in red blood cells called glucosylated phosphatidic acid.
- Sulfoglycoglycerolipids: These compounds contain a sulfur atom and are proposed to be localized to acidic membranes (surface membrane strongly acidic). Sulfolipids are shown to be present in the thylakoid membranes of plants within the photosynthetic membranes.
References
- "Glycolipids". Nature. Nature Publishing Group. Retrieved May 2016.
- Yu et al., R.K. Yu, Y. Suzuki, M. Yanagisawa. Membrane glycolipids in stem cells. FEBS Lett., 584 (2010), p. 1694
- C. Neil Hunter, Fevzi Daldal, Marion C. Thurnauner, J. Thomas Beaty. Advances in Photosynthesis and Respiration, Vol. 28. The Purple Phototrophic Bacteria. Springer. 2009.
- Gillard BK, Thurmon, LT, Marcus DM (1993) Variable subcellular localization of glycosphingolipids. Glycobiology 3: 57-67. 5. Edidin M (2003)
- Simons K, Toomre D (2000) Lipid rafts and signal tranduction. Nat Rev Mol Cell Biol 1: 31-41.
- Brown DA, London E (2000) Structure and functions of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275: 17221-17224.
- Shaul PW, Anderson RG (1998) Role of plasmalemnal caveolae in signal transduction. Am J Physiol 275: 843-851.
- Marrow MR, Singh D, Lu D, Grant CW (1995) Glycosphingolipid fatty acid arrangement in phospholipid bilayer: Cholesterol effects. Biophys J 68: 179- 186
- Kojima N, Hakomori S (1991) Cell adhesion, spreading, and motility of GM3- expressing cells based on glycolipid-glycolipid interaction. J Biol Chem 266: 17552-17558.
- Boggs JM, Menikh A, Rangaraj G (2000) Trans interaction between galactosyl ceramide and cerebroside sulphate across opposed bilayers. Biophys J 78: 874-885.
- Schnaar RL (2004) Glycolipid-mediated cell-cell recognition in inflammation and nerve regeneration. Arch Biochem Biophys 426: 163-172.
- Malhotra R (2012) Membrane Glycolipids: Functional Heterogeneity: A Review. Biochem Anal Biochem 1:108. doi:10.4172/2161- 1009.1000108
- Hakamori SI (2000) Cell adhesion, recognition and signal transduction through glycosphingolipid microdomain. Glycoconj J 17: 143-151.
- Jordan S, Rodgers W (2003) T-cell glycolipid-enriched membrane domains are constitutively assembled as membrane phases that translocate to immune synapses. J Immunol 171: 78-87.
- Auge N, Andrieu N, Negre-Salvayre A, Thiers JC, Levade T, et al. (1996) The sphingomyelin-ceramide signaling pathway is involved in oxidized low density lipoprotein-induced cell proliferation. J Biol Chem 271: 19251–19255.
- De Maria R, Lenti L, Malisan F, d’Agostino F, Tomassini B, et al. (1997) Requirement for GD3 ganglioside in CD95- and ceramide induced apoptosis. Science 277: 1652-1655.
- Nagatsuka Y et al., FEBS Lett 2001, 497, 141
- Benson AA et al., Proc Natl Acad Sci USA 1959, 45, 1582

