5.13: Membrane X-ray Scattering
( \newcommand{\kernel}{\mathrm{null}\,}\)
Membrane scattering encompasses a large variety of methods for characterizing lipid membranes. For example, a few popular techniques include X-ray or neutron reflectivity and diffraction, Brewster angle microscopy, ellipsometry, X-ray interferometry, infrared reflection-adsorption spectroscopy, vibrational sum frequency generation spectroscopy, and any electron scattering techniques such as SEM or TEM [3][4]. These methods can be used with a variety of different model membranes types (described briefly below). For the sake of brevity, this article will focus on just small angle X-ray and neutron reflectivity and small angle X-ray diffraction.
The motivation behind X-ray and neutron reflectivity and X-ray diffraction is mostly to characterize the structure of the lipid membrane such as the membrane thickness, unit cell organization, or where in the membrane proteins prefers to reside. For x-ray and neutron scattering, measurements are performed using flat model membranes that are either at an air-water interface or on a supported substrate. This article will give a brief overview of common membrane scattering techniques and give some background information on flat model membranes.
**note to reader: the text editor software on this website does not support the symbols for greek letters, so in the text lowercase ‘A’ and the symbol for alpha will appear the same. Fortunately I believe that, using the context of the sentence, one should be able to determine the meaning of the variables in this introduction, although I sincerely apologize for any inconvenience.
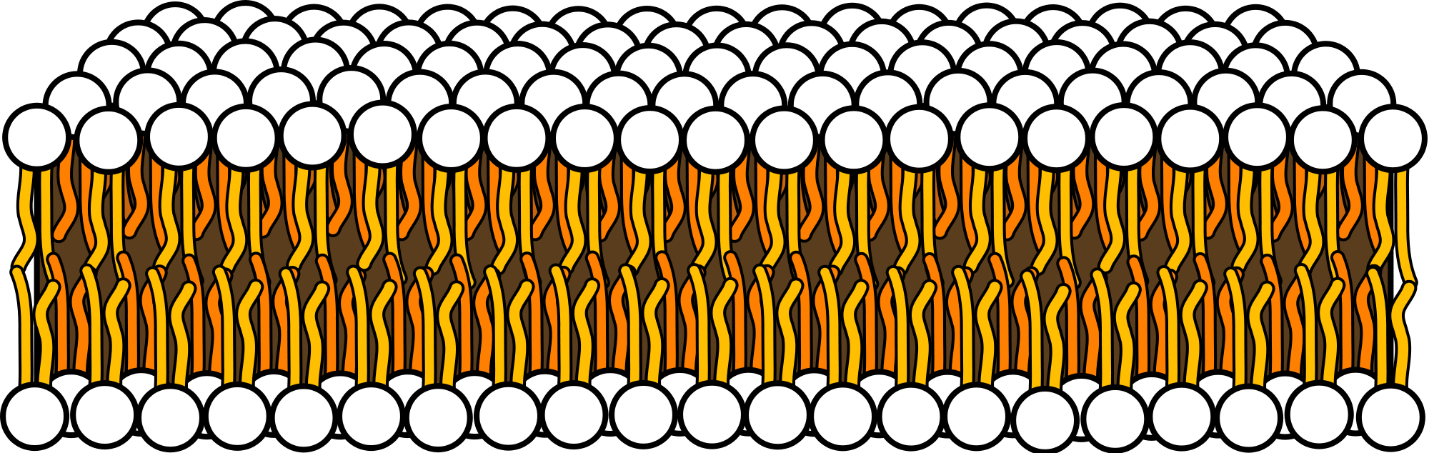
Model Membranes
Due to the enormous complexity of real biological cells and plasma membranes, researchers often use simplified model memsbranes to study specific aspects of how biological membranes function or to make the membranes easier to measure. Importantly, model membranes do not entirely behave like their natural counterparts, and act as more of a guide showing what behaviors are most likely exhibited in real membranes. In addition, different types of model membranes can act differently as well; this paper by the Kuhl Lab at UC Davis explores some of these structural differences using X-ray diffraction [2].
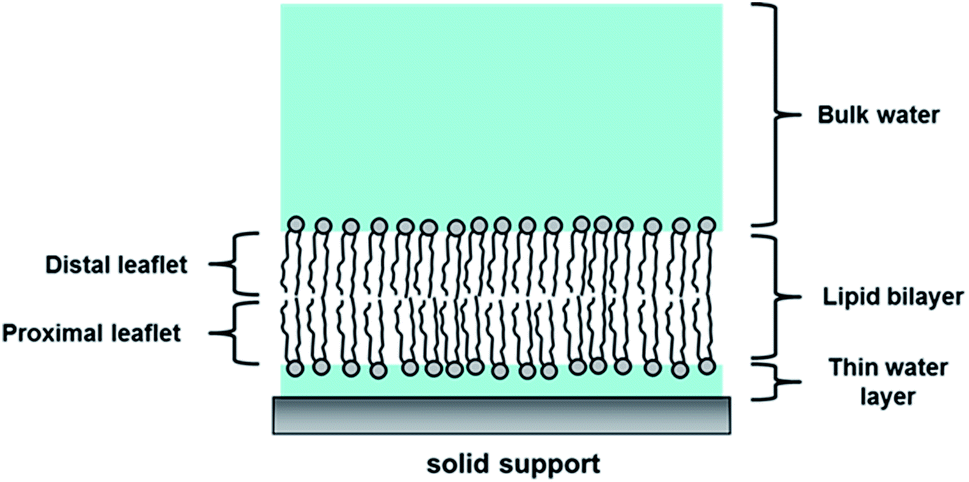
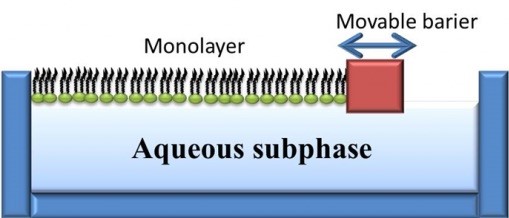
For small angle scattering experiments, only model membranes with flat surfaces can be used, which include monolayer membranes, supported bilayer membranes, and supported multilayer/multilamellar membranes. This is because the incoming beam is approaching the sample horizontally and has a much longer footprint due to it’s angle of incidence being approximately 1⁰.
Scattering Characterization Techniques
Despite being only a few nanometers thick, the bilayer is composed of several distinct chemical regions through its cross-section. Over the past several decades neutron reflectivity, X-ray reflectivity, and X-ray diffraction have been important for characterizing the phases, interactions, and nanostructure of the membrane.
Small Angle X-ray Scattering SAXS (or X-Ray Reflectivity)
Small angle X-ray Scattering (SAXS), or X-ray reflectivity, is a technique that can measure the average electron density of a thin surface layer. The technique can resolve the average height of a monolayer with sub-angstrom resolution, but has the limitations of only working in the out of plane z-direction and only being able to show an average data from a relatively large sample area. One of its advantages, however, is unlike GIXD (described next), SAXS is not limited to only working with ordered phases and can determine the positions of both the head group and the acyl chain tail group of phospholipid molecules. In addition, SAXS has the advantage that it can be done (although perhaps not easily in many cases) with non-synchrotron x-ray sources of still decently high X-ray intensity (such as a rotating or liquid anode x-ray source).
The basic concept behind SAXS is that incoming X-rays will scatter off electrons in a sample (in this case the model membrane). Because the membrane creates an interface, one can use Snell’s and Fresnel’s laws to predict where and with what intensity the X-rays will scatter in the out of plane direction. This scattering intensity turns out to be a function of the average electron density in the z-direction. Thus, to determine the electron density of a sample, a model is made of the samples electron density and refined until it matches the reflectivity data.
To start with, a wave incident on a perfectly sharp interface will reflect or refract according to Snell’s law (which has both a sine and a cosine part) where aI and aR are the incident and reflected intensities:
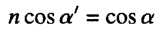
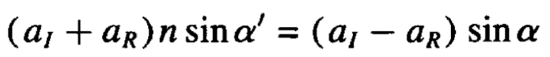
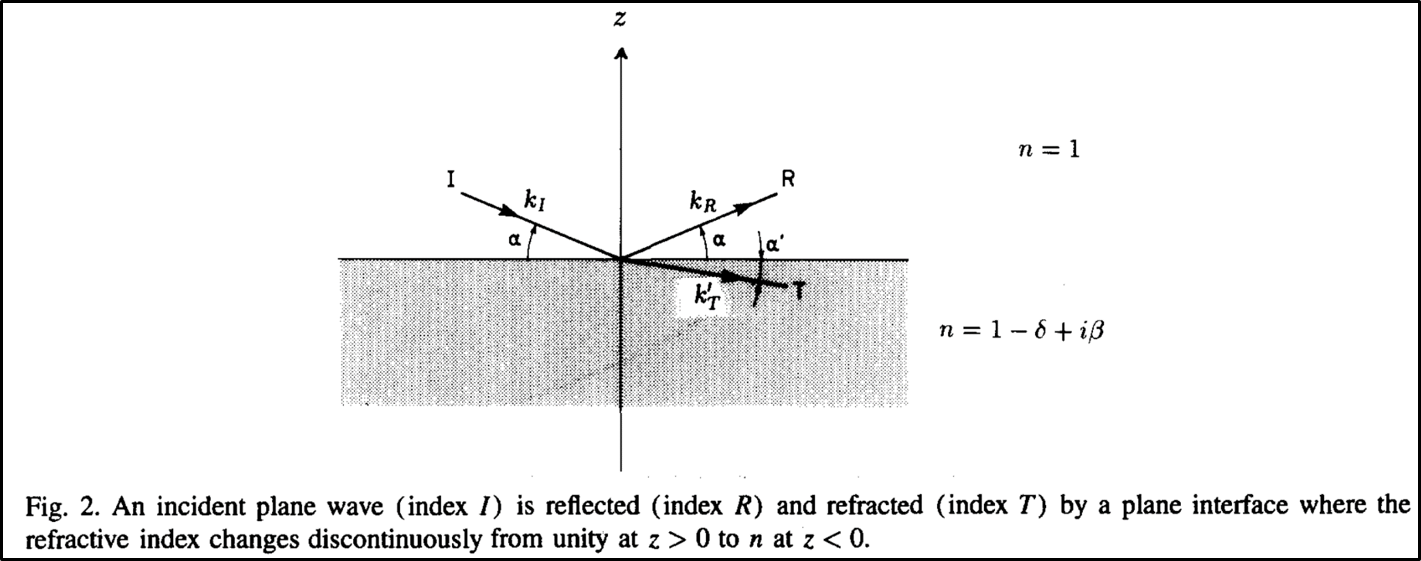
The angle of the reflected beam is also commonly described using the out of plane wavevector transfer qz, which is the difference between the reflected and incident beams, as shown below.
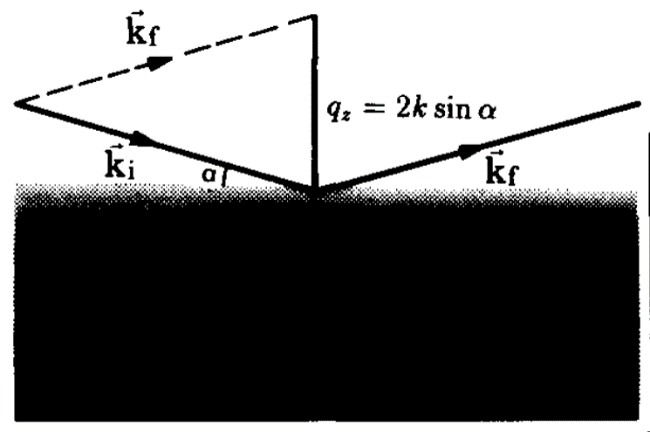
One of the peculiar and important behaviors associated with reflection is as the angle α gets smaller, more and more of the beam is reflected instead of refracted until eventually at a critical angle αc the beam is entirely reflected. For lipid membranes this angle is on the order of about 1⁰. The definition of the critical angle for total reflection is:
α2c≡(4πk2)|ρav|
This can easily be visualized in the graph below, in which as the angle (given as the as the angle divided by the critical angle, which is equal to the wavevector transfer divided by the wavevector transfer at the critical angle) decreases, the reflected intensity increases. The graph also serves to show how non-ideal adsorption behavior can affect the reflected beam intensity even below the critical wavelength.
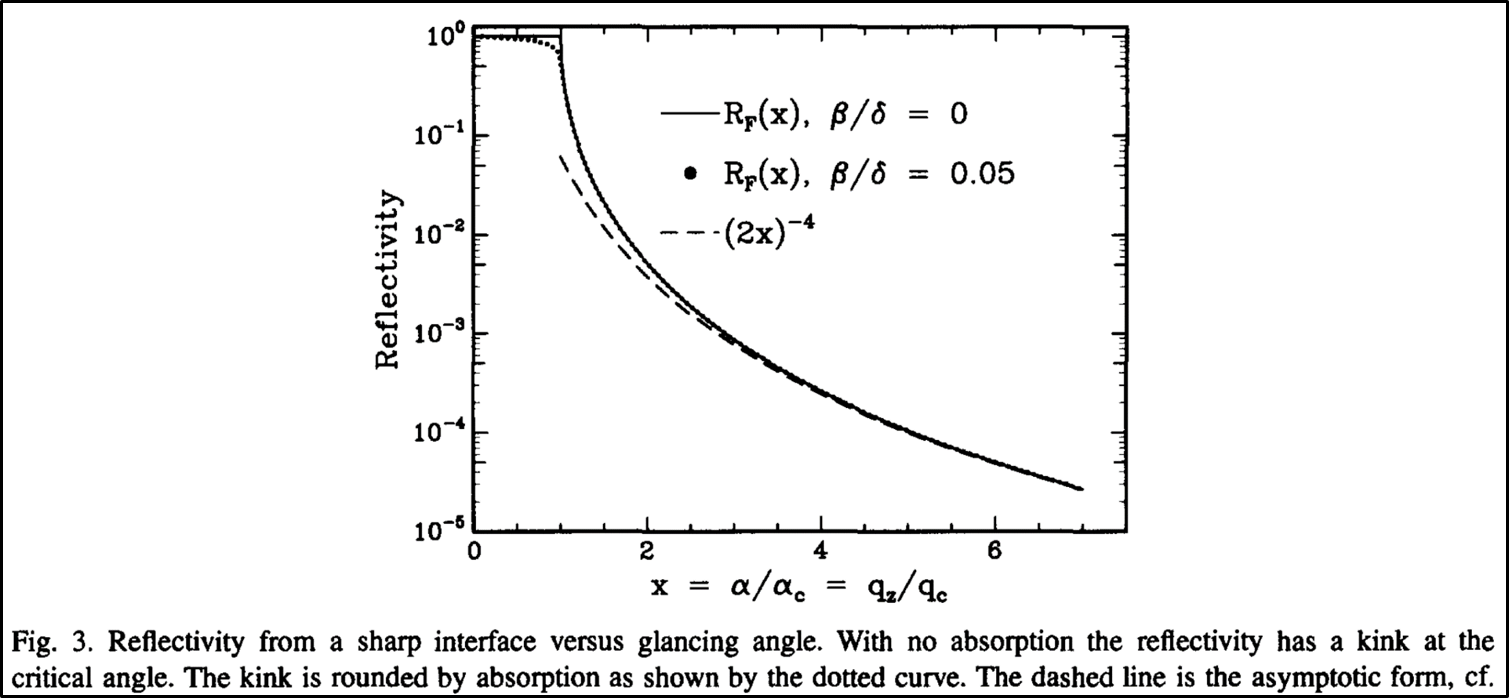
It is important that experiments are carried out close to this critical angle in order to achieve a high enough reflectivity that one can obtain a meaningful signal from their measurements.
Fresnel’s law, the next important element of reflectivity, can be derived using Snell’s law, the definition of the critical angle, and the complex equation for the index of refraction. It can be given as:
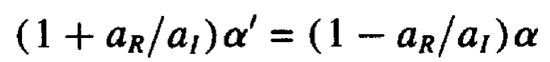
or
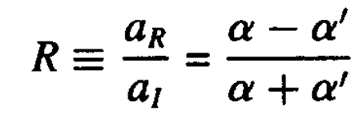
Where R is defined as the ratio between the incident and reflected beam intensities. The reflectivity then, also known as the Fresnel reflectivity, is given as the square of the magnitude of R.
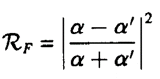
When actually measuring the reflectivity of a sample, these two values are extremely important because the reflected intensity of an X-ray beam off a sample is recorded as R/RF.
In reality, because interfaces are not ideally sharp, the Fresnel reflectivity and Fresnel’s law as shown above don’t apply as they are. The kinematic approximation accounts for such non-ideal behavior and gives the modified Fresnel reflectivity as:
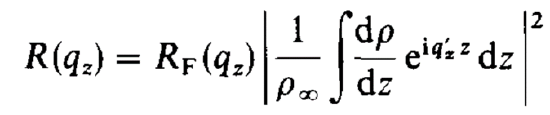
This equation is also used to determine how the electron density ρ determines the observed reflected intensity. By building an electron density model, one is able to work backwards using this equation to fit their electron density to the observed reflection, and thus build an accurate model of the average electron density of their sample in the z-direction.
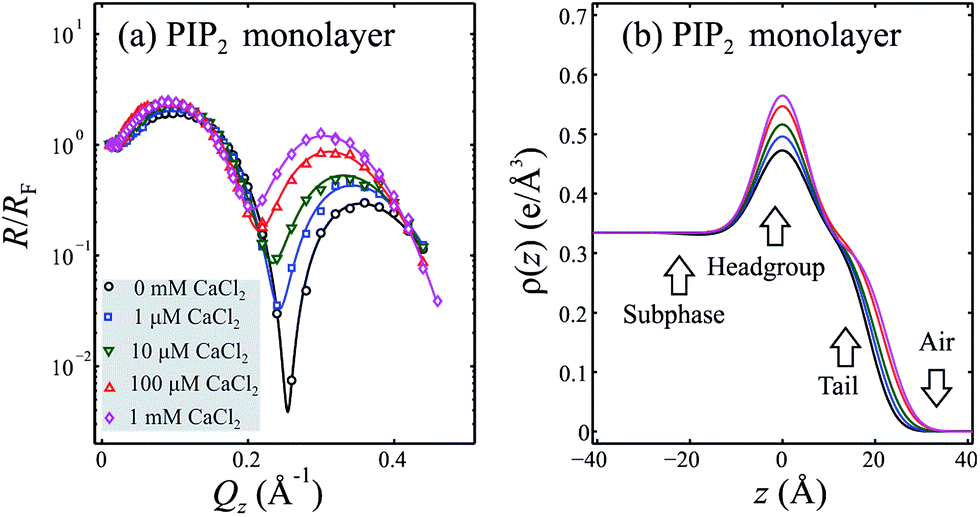
For reference, a reflectivity spectrum and the electron density profile it was matched with are shown below. The data is for a lipid monolayer in a Langmuir trough. In the electron density profile, the lipid headgroups, the acyl chain tails, and the location of the air and water interfaces can all be determined.
Grazing Incidence X-ray Diffraction (GIXD)
GIXD, also called small angle X-ray diffraction, is another technique that uses the small angle approach. In addition, because of the especially high X-ray intensity needed to perform diffraction experiments on lipid membranes, GIXD studies are most often carried out at synchrotron sources.
GIXD can resolve atomic arrangement and unit cell dimensions (to the nearest 0.1 angstrom) of lipid membranes and can measure the tilt angle of the lipid tails to within a few degrees. As the only technique that can measure the lateral organization of the lipid membrane on the nanoscale, it has become extremely useful for investigating the controversial lipid raft theory.
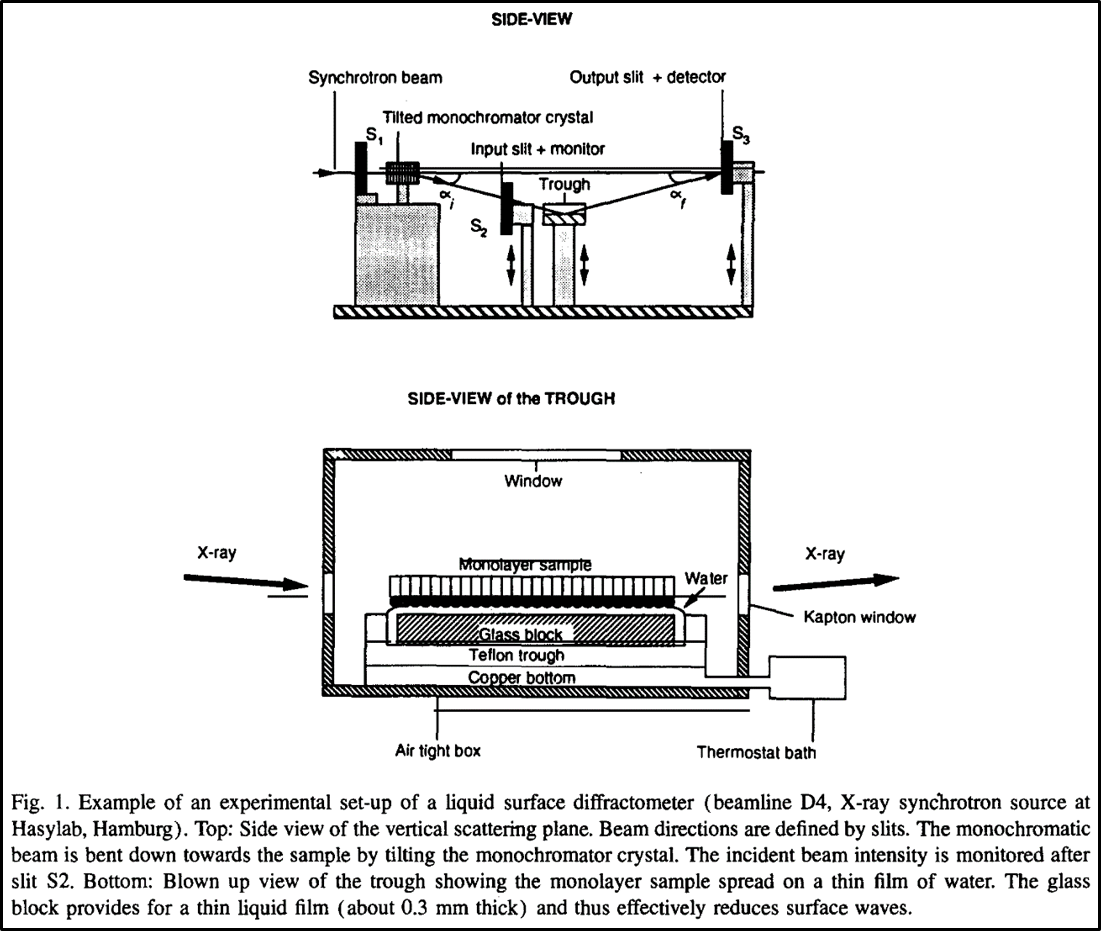
Figure 5.13.8: A clip from a review article (mentioned in further reading) showing an experimental setup for GIXD off a monolayer membrane at a synchrotron facility [7]
However, GIXD is limited in that a high degree of order is needed for diffraction, and even the ordered phases of lipid membranes only form a hexatic phase (a liquid crystalline phase having a high degree of short range order but no long range order). This leads to many of the limitations this technique faces. GIXD cannot “see” the lipid headgroups because only the acyl chain tails are ordered enough to diffract. Even then, because of acyl chain rotation and the lack of long range order, diffraction can only be observed from the entire lipid tail units and not from the individual atoms composing the lipid tails like in most materials. The fact that only acyl chains and not the individual atoms diffract causes monolayer and bilayer (but not multilayer) lipid membranes to only exhibit 2-Dimensional diffraction behavior; i.e. instead of observing cubic or orthorhombic unit cells, one would observe square or rectangular unit cells in a lipid membrane.
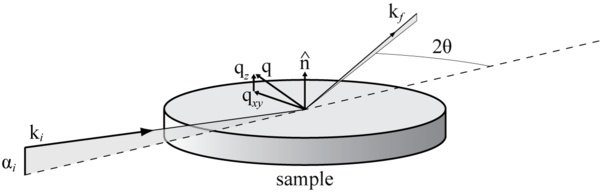
When GIXD is carried out, the incident X-rays approach the sample with a wavevector ki at an angle αi smaller than the critical angle for total reflection αc. Because total reflection occurs, when the X-ray makes contact with the sample it creates an evanescent wave in the top ~10 nm of the sample instead of penetrating deeper. This means that the technique is extremely surface sensitive and able to measure only the top 10 nm of a sample, which is extremely useful for studying lipid membranes (bilayers are approximately 5-7 nm thick). The X-rays then diffract off the Miller planes within a sample with a new wavevector kf. The difference between kf and ki is called the wavevector transfer, denoted by q. q can then be further divided into the in-plane and out-of-plane wavevector transfers, qxy and qz respectively. qz is related to the out of plane angle as shown in the X-ray reflection section, while qxy is related to the in-plane 2θ angle used in standard X-ray diffraction experiments by (with λ as the X-ray wavelength):
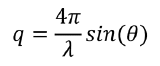
After the beam is diffracted, the resulting diffraction pattern is collected by an X-ray sensor and the background noise is subtracted (which can, in some cases, be extremely difficult). Once the background is subtracted, a pattern similar to the one below can be analyzed.
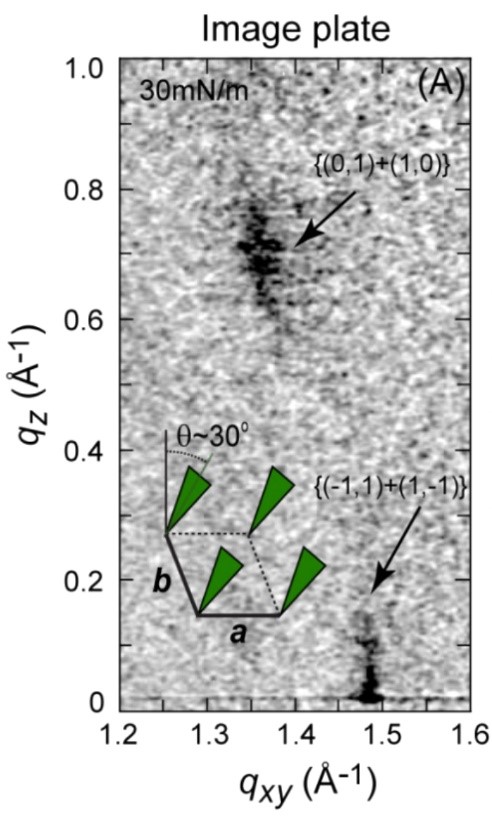
To analyze the pattern, it is integrated over qz to create the Bragg peak spectrum and integrated over qxy to create the Bragg rod spectrum [17]. However, it is important to note that compared to a standard X-ray diffraction pattern, a GIXD image plate only covers an extremely small fraction of the 2θ spectrum, and thus very few peaks are generally observed.
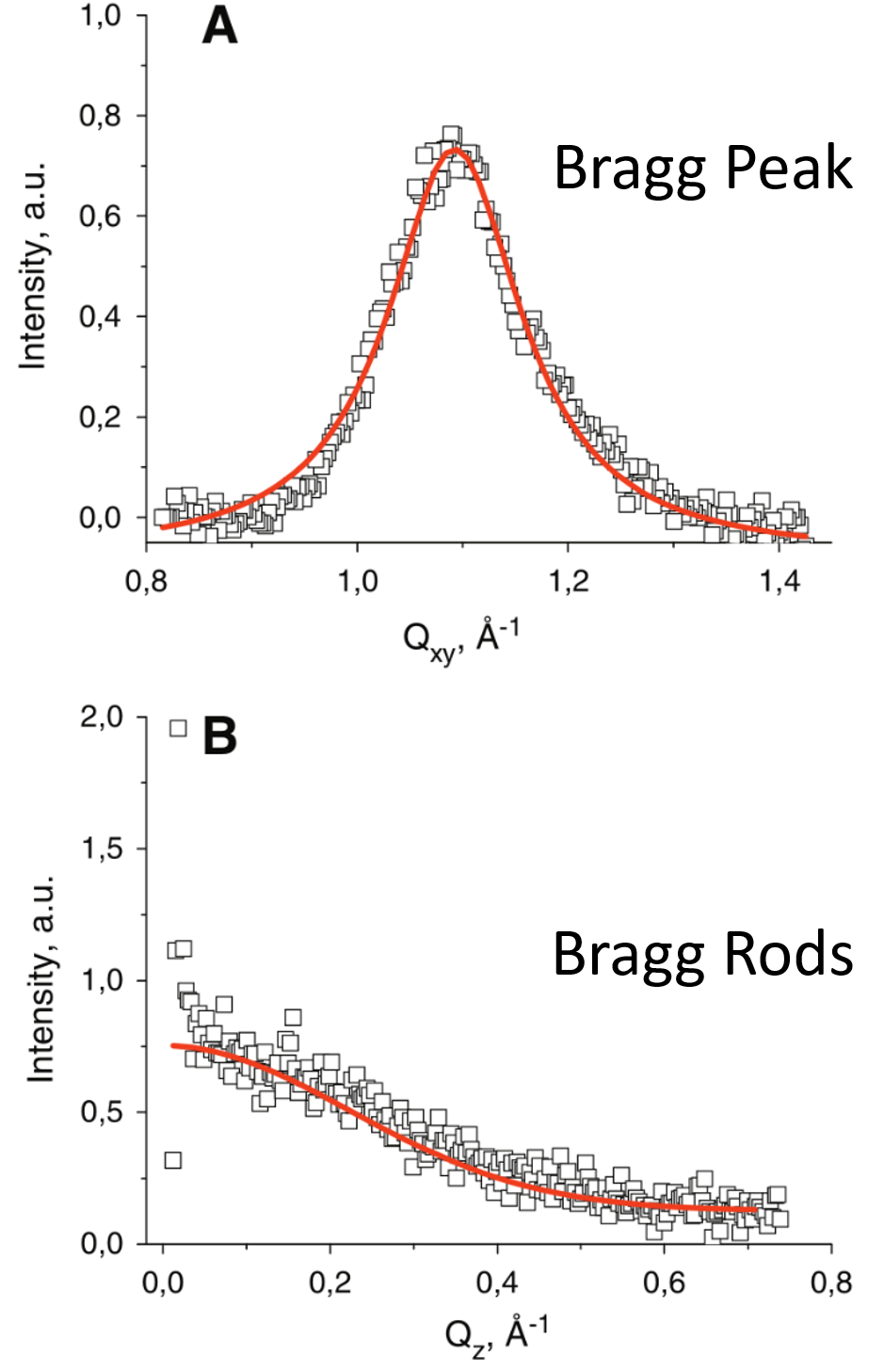
The Bragg peaks can be indexed using Bragg’s law or another standard method (such as an Ewald sphere) of analyzing diffraction patterns. Bragg rods for lipid membranes are generally more difficult to analyze directly and are investigated by making a model of the diffracting structure which includes the acyl chain tilt) and measuring how well the predicted diffraction from the model matches the observed Bragg rod pattern. From these methods, the symmetry and size of the unit cell can be calculated.
In addition, the in-plane coherence length Lc, which is a measure of how far the short range crystalline order extends, can be calculated using the full width half max (fwhm) of the Bragg peak:
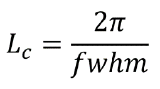
Neutron Reflectivity
Neutron scattering generally follows the same mathematics and experimental methods as X-ray scattering. However, the basic principle of it and therefore the information it can extract is slightly different, because instead of scattering off the electrons in a sample, neutrons scatter off the atomic nuclei in a sample.
Therefore neutrons, unlike other common scattering sources, will interact differently with separate isotopes of the same atom. This is especially evident for hydrogen and deuterium, which is convenient because all the organic molecules normally present in a biological membrane have many hydrogen groups. Thus, it is common to label proteins, water, or other items of interest with deuterium and use neutron reflectivity to find their location in the membrane [5].
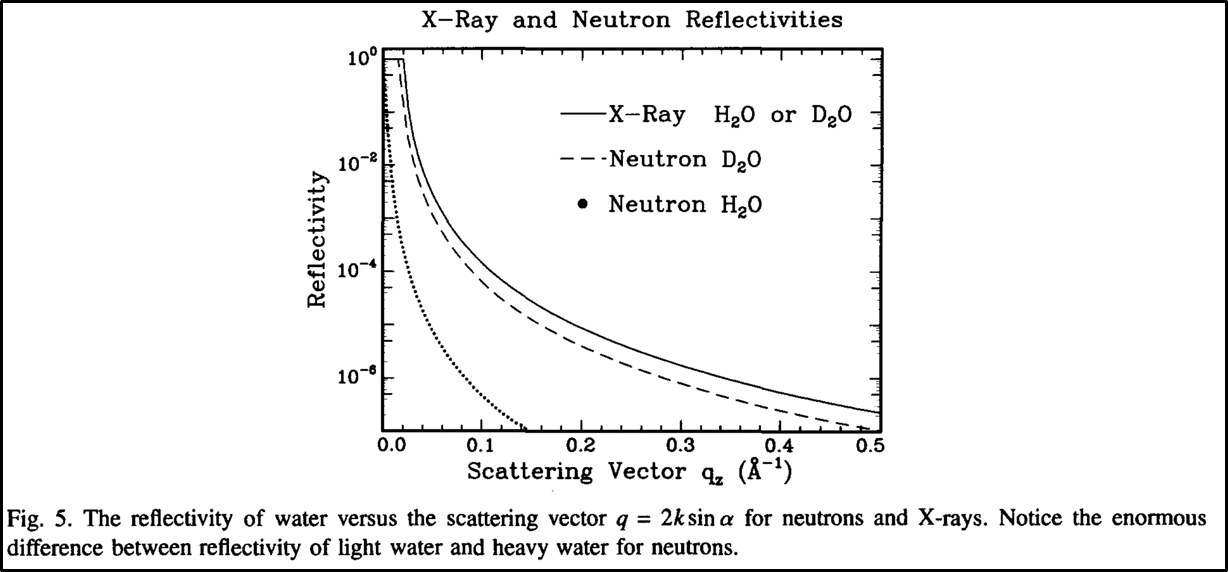
One of the common types of studies neutron reflection is used for is to determine how viral proteins can break through cell membranes. By using deuterium water on one side of the membrane and hydrogen water on the other side of the membrane, scientists can observe as the water slowly makes its way across the membrane as more proteins are added [21]. This is a good example of how neutrons are generally used to observe very specific phenomena, usually through deuterium labeling.
Unfortunately, one of the most difficult obstacles associated with neutrons is that neutron sources are very low intensity. This generally means it takes much longer to run neutron scattering experiments than equivalent X-ray scattering experiments. However, it also makes neutron diffraction experiments on lipid membranes extremely difficult and current membrane neutron diffraction experiments are extremely rare and can only use multilayer lipid model membranes; these types of membranes have much stronger diffraction signals than monolayers or bilayers [6].
Beam Sources
Membrane scattering techniques all require some type of wave or particle, such as a beam of X-rays or neutrons, to be accelerated towards a sample. It's effect is then measured and this is how the properties of the sample are determined. Often understanding the sources of the particles or waves is often an integral part of understanding the experimental method.
X-ray Sources
Because X-rays scatter off electrons and most lipid membranes consist almost entirely of lighter elements, meaning they have a low electron density, it can be difficult to get a significant signal to noise ratio with X-ray scattering on lipid membranes. Therefore, it is very common to use synchrotron X-ray sources so that, by using a much higher X-ray intensity, it will be easier to determine the meaningful data from the background noise. In addition to having an extremely high X-ray intensity (meaning a high flux of X-ray photons), these also have the advantage of variable photon energies. In addition, and also due to the high intensity, synchrotrons are able to finish experiments much faster than other X-ray sources. [1]
Synchrotrons, which are huge facilities with their electron storage rings often on the order of a kilometer in diameter, operate by accelerating electrons or positrons to nearly the speed of light and then forcing them to oscillate. In the process of oscillating, the electrons emit X-rays that can be monochromated (usually by diffraction from germanium single crystal) and then focused on the sample using X-ray optics.
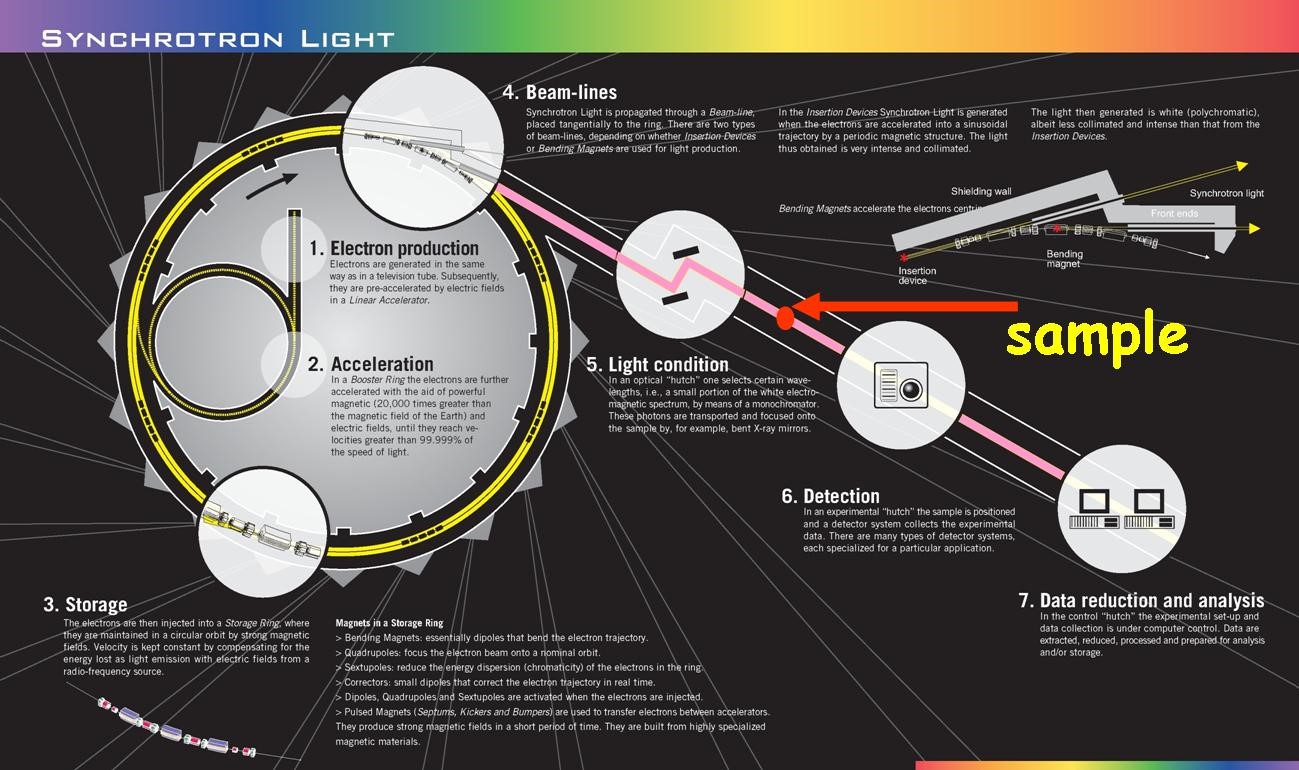
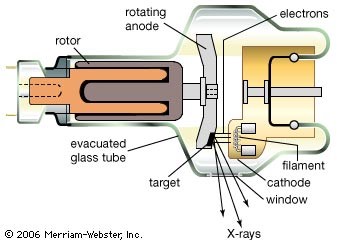
For membrane reflectivity experiments that don’t need a high intensity of X-rays, rotating anode or liquid anode X-ray sources can also be useful (however, previous generations of X-ray sources such as standard sealed tube X-ray sources may not have high enough intensities). For example, a monolayer X-ray reflectivity measurement could likely be carried out with one of these because monolayers give a relatively large signal to noise ratio and their sample chambers have very small X-ray absorption rates.
Neutron Sources
Neutrons for characterization are produced via one of two methods: nuclear fission reactions or spallation. Nuclear fission sources are the older generation, and operate similarly to a nuclear fission reactor: a heavy, radioactive element is fissioned in a chain reaction that eject neutrons from the atomic nuclei, and this chain reaction is moderated (slowed/controlled) by heavy water. In a fission source, the wavelength (energy) of the incoming neutrons is controlled by the temperature of the moderating medium. [12]
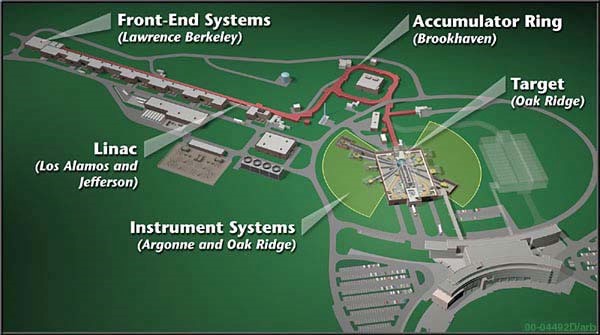
Spallation sources are now preferred over neutron sources because they have a much higher neutron intensity (number of neutrons emitted from a source) than the fission sources and can be much more easily controlled; while a uranium fission source emits 3 neutrons per event, a spallation source can emit 20-30 per event! Because one of the major limitations for neutron scattering is the low source intensity, this newer generation of neutron sources is an important step on the way to making neutron scattering experiments more widely applicable. In spallation, metal targets are bombarded by high energy protons, and every time a nucleus breaks down it releases neutrons in a spallation event. Because of the much higher intensity of neutrons produced by spallation sources, monochromators or time of flight filters (wavelength filtering techniques) can be used to limit the source to a very narrow wavelength distribution, which is very helpful for scattering experiments (a higher intensity source is needed because intense filtering can greatly decrease the intensity of a beam).
In addition, something to be aware of when using a neutron scattering facility is that neutrons have the potential to make your sample radioactive. If the sample has a high enough radioactivity, the lab the neutron source is located at may deem it unsafe to handle and will either wait for a period of months to years to return it or destroy it as radioactive waste.
Further Reading
For a deeper look into X-ray and neutron scattering, there are three main sources that were the most important sources of information for this work.
The first is reference [1], ‘Elements of Modern X-ray Physics’, by Als-Nielsen et al. It is a comprehensive book on X-ray scattering and diffraction using synchrotron sources and touches on neutron theory as well.
The next is a large review article [7], again by Als-Nielsen et al., ‘Principles and applications of grazing incidence x-ray and neutron scattering from ordered molecular monolayers at the air-water interface.’ While being much shorter than a book, this article is still a fairly comprehensive review of the subject.
The last is by Kjaer [8], and is an incredibly abbreviated introduction to X-ray scattering and reflectivity, and would be most useful for those already familiar with the concept of an inverse lattice.
Best of luck to all in your further studies!
Note to further contributors
For all future contributors, please continue posting descriptions of even more membrane scattering techniques. There are still a lot of membrane scattering methods left to describe.
References
- J. Als-Nielsen, D. McMorrow, Elements of Modern X-Ray Physics, Wiley (2001), p. 83.
- Watkins, Erik B., et al. "Equilibrium or quenched: fundamental differences between lipid monolayers, supported bilayers, and membranes." ACS nano 8.4 (2014): 3181-3191.
- Gözen, Irep, and Aldo Jesorka. "Instrumental methods to characterize molecular phospholipid films on solid supports." Analytical chemistry 84.2 (2012): 822-838.
- Lösche, Mathias. "Surface-sensitive X-ray and neutron scattering characterization of planar lipid model membranes and lipid/peptide interactions." Current topics in Membranes 52 (2002): 117-161.
- Zheng, Songyan, et al. "Structural studies of the HIV-1 accessory protein Vpu in Langmuir monolayers: synchrotron x-ray reflectivity." Biophysical journal 80.4 (2001): 1837-1850.
- Harroun, T. A., et al. "Neutron diffraction and Vitamin E." Journal of Physics: Conference Series. Vol. 251. No. 1. IOP Publishing, 2010.
- Als-Nielsen, Jens, et al. "Principles and applications of grazing incidence x-ray and neutron scattering from ordered molecular monolayers at the air-water interface." Physics Reports 246.5 (1994): 251-313.
- Kjaer, Kristian. "Some simple ideas on x-ray reflection and grazing-incidence diffraction from thin surfactant films." Physica B: Condensed Matter 198.1-3 (1994): 100-109.
- Villarreal, Marina Ruiz; username: LadyofHats. File: Phospholipids aqueous solution structures.svg; Wikimedia Commons. 6 November 2007. commons.wikimedia.org/wiki/File%3APhospholipids_aqueous_solution_structures.svg
- Alessandrini, Andrea, and Paolo Facci. "Phase transitions in supported lipid bilayers studied by AFM." Soft matter 10.37 (2014): 7145-7164.
- Ciumac, Daniela. “Langmuir Trough.” SNAL Marie Curie ITN Network. 28 Oct. 2014. Accessed: 7 Jun. 2017
- Kovnir, Kirill. “Lecture 7: Neutrons, TEM.” Chemistry 217. UC Davis, California. 5 Aug. 2017
- Username: Horakcm. File: Sns-facility-design.jpg; Wikimedia Commons. 3 Nov. 2011
- “What is a Synchrotron.” ALBA. Accessed: 7 Jun. 2017. Retrieved from https://intranet.cells.es/AboutUs/WhatIs
- Usernames: Mohamed.ah; drewhunt et al. “Physics of The X-ray Tube.” 13 Oct. 2012. Accessed 7 Jun. 2017
- Username: Jaknelaps. File: Grazing incidence diffraction GIXD.png; Wikimedia commons. 7 April 2014. commons.wikimedia.org/wiki/File:Grazing_incidence_diffraction_GIXD.png
- Watkins, Erik B., et al. "Equilibrium or quenched: fundamental differences between lipid monolayers, supported bilayers, and membranes." ACS nano 8.4 (2014): 3181-3191.
- Wydro, Paweł, Michał Flasiński, and Marcin Broniatowski. "Grazing Incidence X-ray Diffraction and Brewster Angle Microscopy studies on domain formation in phosphatidylethanolamine/cholesterol monolayers imitating the inner layer of human erythrocyte membrane." Biochimica et Biophysica Acta (BBA)-Biomembranes 1828.6 (2013): 1415-1423.
- Kjaer, Kristian. "Some simple ideas on x-ray reflection and grazing-incidence diffraction from thin surfactant films." Physica B: Condensed Matter 198.1-3 (1994): 100-109.
- Graber, Z. T., et al. "Competitive cation binding to phosphatidylinositol-4, 5-bisphosphate domains revealed by X-ray fluorescence." RSC Advances 5.129 (2015): 106536-106542.
- Choi, D., et al. "Insertion mechanism of cell-penetrating peptides into supported phospholipid membranes revealed by X-ray and neutron reflection." Soft Matter 8.32 (2012): 8294-8297.

