2.3: The Electromagnetic Spectrum
( \newcommand{\kernel}{\mathrm{null}\,}\)
- You will be able to rank the different bandpasses in the EM Spectrum by energy, frequency, and wavelength
On their way to a concert, three students decide to listen to the radio.
- Angela: “I wonder if radio telescopes work the same way as the radio we’re listening to. You know, by picking up sound waves that we can hear.”
- Brianna: “I don’t think so, there’s no air in space for the sound waves to travel through, so you wouldn’t be able to get any signals.”
- Callie: “Right, radio waves are light waves. The radio is really detecting light waves in the radio portion of the spectrum and converting them into sound waves that we can hear.”
Do you agree with any of these students, and if so, whom?
Angela
Briana
Callie
None
Explain.
While much can be learned from observing objects in visible light—the kind of light we see with our eyes—there is a tremendous amount of additional information carried only by other kinds of light. Over the past half-century or so, astronomers have learned to observe the Universe not only in visible light, but in all bands of the electromagnetic (EM) spectrum, from low-energy radio waves all the way up to high-energy gamma-rays. Figure 2.2 shows the (EM) spectrum.
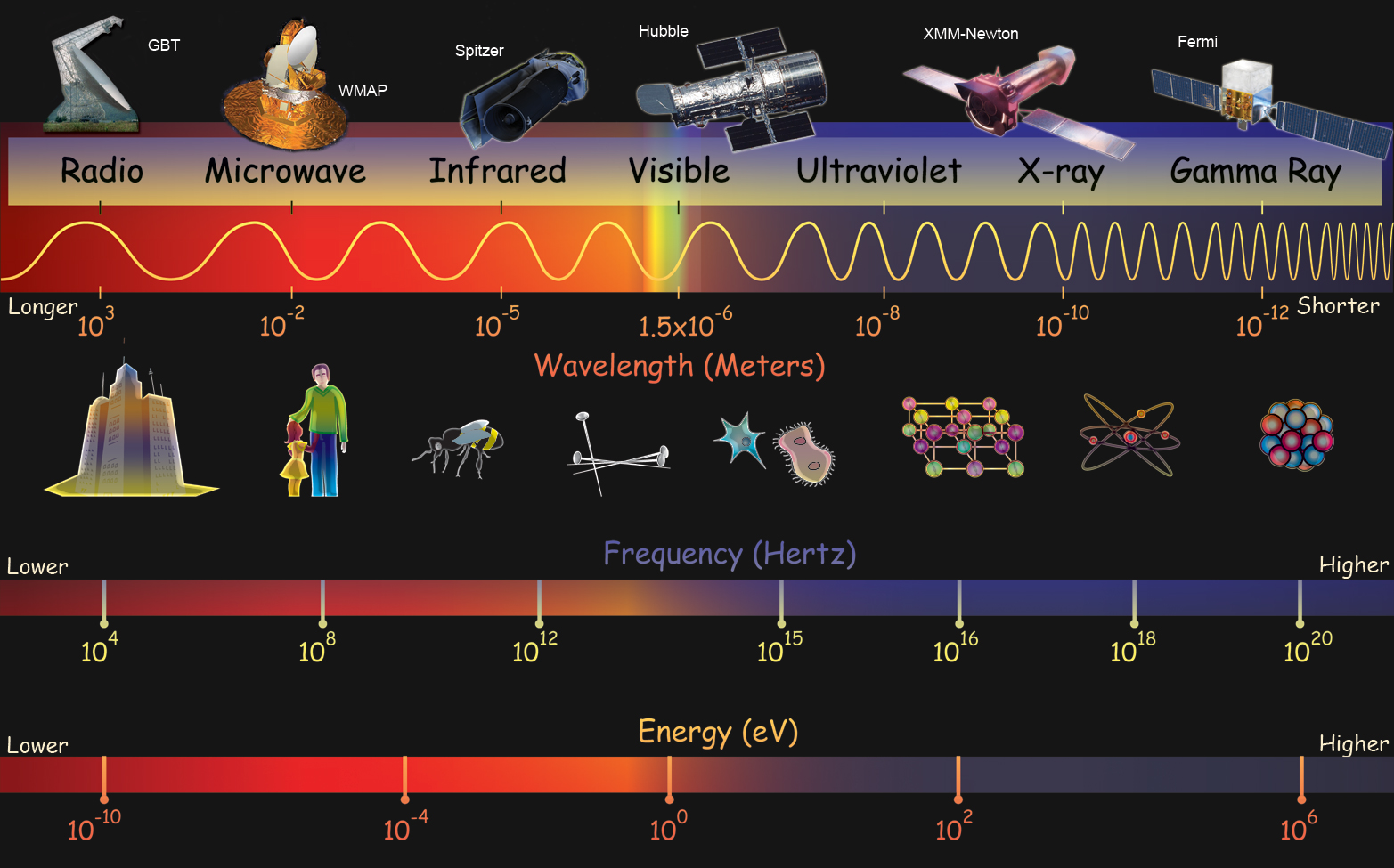
The EM spectrum, of which visible light is a tiny part, is divided into somewhat arbitrary regions, or bands, that are based on how the light in that region is measured. These different methods of detecting or measuring the light result from the different wavelengths or frequencies of the light in each band. To give you an overview, and to put some useful labels on what we are discussing, we will briefly describe the different bands of the EM spectrum. It turns out that most portions of the EM spectrum are not visible to our eyes.
- Radio—At the longest wavelength end of the EM spectrum are the radio waves. This is the kind of light used to carry radio signals to a stereo in your car and to your television (if you receive broadcast TV and not cable). Are you surprised to find that radio is a kind of light, and not a kind of sound? Many people are surprised by this fact when they first learn about it. Since astronomical radio waves have such long wavelengths, they are usually detected using telescopes that look like big dishes. There is no limit to the length of radio waves (on the long end), but the shortest radio waves have lengths of about a meter.
- Microwaves—At the high-frequency end of the radio spectrum are microwaves. They are the types of waves used in microwave ovens, where they interact with the water molecules in your food to heat it up. They also carry the signals used by cell phones. Although the prefix “micro” usually means “one-millionth,” microwaves have typical wavelengths of centimeters (one-hundredth of a meter) to millimeters (one-thousandth of a meter).
- Infrared—At slightly higher energies than microwave is infrared light. Infrared (IR) light is between microwaves and the red end of the visible light spectrum (infra is a prefix that means “below” — IR has a frequency below that of visible light). IR is sometimes called heat radiation, but this is a misnomer. As we will learn, all kinds of EM radiation are emitted by warm objects. But IR is emitted predominantly by objects we consider warm. Such objects include hot coals and warm-blooded animals (including people). Long-wavelength IR is also emitted by telescopes themselves. IR light has enough energy that it is not usually measured as a wave, but rather as a particle. IR photons have wavelengths in the range of hundreds to 1 or 2 microns (millionths of a meter).
- Visible Light—Next is visible light, also known as optical light. We perceive many different colors of visible light, which correspond to different frequencies of the light. At the low-frequency end is red, followed by orange, yellow, green, blue, and violet. One common mnemonic used to remember these colors in the proper order is ROY G. BIV (where indigo has been inserted to make the phrase easier). Check it out the next time you see a rainbow. Optical astronomy has been done using telescopes made of mirrors and lenses since the early 1600s, when Galileo Galilei first used a telescope to study the night sky. Visible light spans the wavelength range 400–700 nanometers (billionths of a meter), or if you prefer, it has wavelengths clustered around half a micron.
- Ultraviolet—Ultraviolet (UV) light is just beyond the blue end of the visible spectrum and it has somewhat higher frequencies than visible light. UV is what gives you a suntan (or sunburn with too much exposure). For the most part, exposure to light from radio through visible is generally without risk (but there are exceptions). At UV, this risk changes; too much exposure to light at UV or higher frequencies can be harmful or even fatal because these particles have enough energy to dislodge electrons from the atoms in your body (in a manner similar to the photoelectric effect ). Although some low-energy UV rays penetrate Earth’s atmosphere, most higher-energy UV does not. Hence, most UV astronomy is done from detectors launched into space. UV photons have wavelengths typically measured in tens to hundreds of nanometers.
- X-rays—X-rays have energies higher than UV. You probably know from visits to the doctor or dentist that x-rays can be used to create images of our insides. These images are not like normal photographs, however. They are really shadows. X-rays can penetrate many materials, but their penetrability depends on the density of the material. Thus, they pass easily through skin and muscles, but less easily through bones and teeth. As a result, x-ray images really just show contrast in the shadows caused by different parts of the body. They can also be used the same way to look into other materials, like machines and machine parts. X-ray images of astronomical objects are very different because the objects are emitting x-ray photons and the telescope is just catching them. Since Earth’s atmosphere absorbs x-rays from space, x-ray astronomy is always done from instruments in space. X-rays have so much energy that we tend to describe them using the energy of individual photons, rather than specifying their wavelengths or frequencies. X-ray energies start at 1 keV (kilo or thousands of electron volts), which are a more convenient measurement than using the SI unit joules. (One eV is equal to 1.6 × 10-19 J.)
- Gamma-rays—The highest energy light is called gamma-rays. At low energies, gamma-rays merge into x-rays. In principle, there is no upper limit to gamma-ray energies; their energies could go to higher and higher values. In practice, we will see there are practical limits to how high the energy of a gamma-ray can go. Gamma-rays are the least familiar kind of light because they are almost completely absent on the Earth’s surface. This is a good thing. Gamma-rays interact so strongly with matter (including the matter comprising the proteins and DNA in our bodies) that a small exposure to them can be harmful, or even fatal. Gamma-ray energies start around 1 MeV (mega or million electron volts). The highest gamma-ray energies that have been directly measured from astronomical objects are in the TeV (tera or trillion electron volt ) range, though indirectly detected gamma rays have been seen with energies more than a million times higher.
Now that you have learned about the different bands of the electromagnetic spectrum, you will do some activities exploring wavelength, frequency, energy, and speed.
A. Wavelength.
Rank the different bands of the EM spectrum in order from shorter wavelength (on the left) to longer wavelength (on the right).
B. Energy.
Rank the different bands of the EM spectrum in order from lower energy (on the left) to higher energy (on the right).
C. Frequency.
Rank the different bands of the EM spectrum in order from lower frequency (on the left) to higher frequency (on the right).
D. Relationships.
1. How did the three rankings you completed compare with one another?
Ladies and gentlemen! Place your bets! Which colors of light do you think travel fastest through the vacuum of empty space?
1. Rank the wavebands: gamma-ray, x-ray, optical, and radio, according to their speeds.
Now that you have made your predictions, press the “start” button to enjoy the race.
2. Describe how the outcome of the race compares with your initial predictions.
3. Explain how the equation that describes the relationship between a wave’s wavelength and frequency (λ = c / f ) relates to the outcome of the race.
In this activity, you will have a chance to practice converting the units of energy for photons using the conversion factor 1 eV = 1.6 × 10-19 J. Sometimes astronomers express energy in keV (103 eV) or MeV (106 eV).
Worked Example:
1. An x-ray photon has an energy of 1 keV. What is its energy in joules?
- Given: E = 1 keV = 1E3 eV
- Find: Energy in joules
- Concept(s): 1 eV = 1.6E-19 J
- Solution: E = 1 keV = 1E3eV × (1.6E-19 J / 1 eV) = 1.6E-16 J
Questions
1. A gamma-ray photon has an energy of 6.63 x 10-13 J. What is its energy in MeV?
MeVShow your work:
Matching.
Above the table are several tiles, each with a specific energy, frequency, or wavelength. Drag and drop each tile into the appropriate row and column on the chart of the EM spectrum until all tiles have been placed. If the tile is placed correctly, a green check mark appears.
If you are having difficulty, you may want to refer to previous activities where you ranked the energies, frequencies, and wavelengths of the various bands of the EM spectrum.
Challenge round: Compute the values.
Once you complete part A, blank tiles will automatically appear in the rest of the chart.
Complete the chart by computing the energies, frequencies, and wavelengths for the remaining tiles and entering the values on the tiles:
- Click the “edit” button, which is the vertical bar on the right-hand side of the tile
- Enter the coefficient into the first small box
- Enter the exponent into the second small box
- Select the appropriate units
- Click the “edit” button again to check your answers
Try at least one example of each type to make sure you understand the calculations.
Show your work here:


