16.1: The Formation of the Lightest Elements
( \newcommand{\kernel}{\mathrm{null}\,}\)
- You will know the overall abundances of the elements.
- You will know how we measure abundances (especially of the lightest elements).
- You will be able to determine which abundances are primordial.
- You will be able to explain how the neutron to proton ratio = 1/7 causes basic proportions of ¾ H and ¼ He.
- You will understand the effect of baryon-to-photon ratio on the abundances of the light elements.
- You will know that the measured abundances of the light elements can all be explained by Big Bang Nucleosynthesis.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
We have seen that the Universe is hotter as we look further back in time. Figure 16.2 shows how the temperature of the Universe changes with time. In this chapter, we will see how conditions in the early moments of existence are shaped by this change in temperature.
.jpg?revision=1&size=bestfit&width=464&height=444)
During a period from about 0.001 seconds to about 3 minutes, when temperatures were billions to millions of kelvin, our ideas of what conditions in the Universe were like are still on fairly firm ground. The relevant physics at these temperatures is well-understood from laboratory experiments, theoretical models, and astronomical observations. We know that the conditions in the first few minutes of the Universe were just right for nuclear fusion to form the very lightest elements: hydrogen, helium, and lithium.
Observations
As we gaze out into the night sky, we wonder where the stuff we are made of came from and whether the rest of the Universe is made of the same stuff as we are. Remarkably, it is possible to answer these questions through observations. We can use spectroscopy to determine the chemical composition of planets, stars, and other objects in the Universe. We accomplish this by looking at their light, using the fact that each chemical element has a unique spectral signature.
As we study the distribution of the chemical elements in the Universe, we can also ask where the elements came from, and why there are different amounts of each. From studies of meteorites, the atmospheres of other planets in the Solar System, the Sun and other stars, as well as the dust and gas between stars and even between us and distant quasars, we have determined the abundance of the various elements in the Universe. Figure 16.3 summarizes our findings in terms of the numbers of each element relative to the others. Today, hydrogen makes up about 74% of the mass (and 92% of the number) of the atoms in the Universe. Helium makes up about 24% of the mass of atoms (8% of the number). The rest, only 2%, is all of the other elements.
In contrast, Earth is made primarily of heavier elements; hydrogen and helium are only tiny constituents in Earth's composition, the surface of which is mostly silicon and oxygen. There are also significant amounts of aluminum, iron, calcium, and a few others. Earth's atmosphere is primarily nitrogen and oxygen, and most living organisms are substantially composed of carbon and its compounds, along with water (hydrogen and oxygen). So hydrogen is only a small fraction of Earth’s mass, and the same is true of helium. Chemically speaking, Earth is highly atypical of the Universe as a whole.

Some of the most intriguing celestial observations are of the lightest elements: hydrogen (H), helium (He), lithium (Li), and the isotope of hydrogen known as deuterium (2H; 1 proton and 1 neutron, often denoted D). We never measure less than about 23% of the mass of atoms being in 4He, whether it is measured in stars, nebulae, or galaxies. We also always see some deuterium- at least 1 part in 100,000 (10-5) compared to regular hydrogen, whether it is measured in the molecules of Jupiter’s atmosphere, the local interstellar medium, meteorites, or distant quasars - despite the fact that deuterium is destroyed inside stars, not produced there. We measure the 3He abundance in nebulae, the oldest meteorites, lunar soil, and the solar wind, and it also has an abundance about 10-5 compared to hydrogen. The same process that destroys deuterium in stars creates 3He, so we have a check on our model for deuterium. The observations of these elements lead us to believe they were formed somewhere other than in stars.
We can also compare the abundance of light elements (such as lithium, beryllium, and boron) in stars to the abundance of the heavy elements (such as iron) in stars. Stars create heavier and heavier elements throughout their lives as the result of nuclear fusion in their cores. When they die, they eject the elements into the surrounding interstellar medium and new stars (and planets) form from the enriched material. This means that stars with a lower abundance of heavy elements must have been created at earlier epochs in the Universe, before many generations of stars had sprinkled the interstellar gas with the products of their nuclear fusion. Stars with a higher abundance of heavy elements must have been formed later.
Figure 16.4 shows the spectra of several stars, each from a different generation. The abundance of heavy elements increases over time; thus, the abundance of heavy elements can be used as a marker for time.

Measurements of solar-like stars (as well as of meteorites and the local interstellar medium) imply an abundance today of about 1 part in a billion (10-9) for 7Li. Starting in the 1980s, astronomers started looking at the abundance of light elements in very old stars, that is, stars with a low abundance of heavy elements such as iron. Stars with fewer heavy elements (indicating older generations of stars), have less 7Li- up to a point. The abundance plateaus at a value of ~10-10 and does not drop as iron abundance continues to drop. The next heavier elements in the periodic table, beryllium and boron, do not show such a plateau, as indicated in Figure 16.5. This result implies that at least some 7Li was present before the oldest stars formed, but heavier elements, such as beryllium and boron were not.

Taking all of the data into account, the primordial abundances by number of atoms of the elements present before the formation of first stars were: 92% hydrogen (1H), 8% 4He, ~10-5 deuterium, ~10-5 3He, and ~10-10 7Li. By mass, this corresponds to about 75% hydrogen (1H) and 25% helium (4He), and only a tiny fraction of the mass in other isotopes.
Models
The observed amounts of the lightest chemical elements, such as hydrogen, deuterium, helium, and lithium, imply that these elements were created early in the history of the Universe before stars formed. This can only have been in the first few minutes, when conditions were hot and dense enough for nuclear fusion to occur. The abundance measurements are consistent with expectations given the relative densities of protons, neutrons, and photons, and the temperatures and densities of the Universe at these early times.
The creation of the lightest elements in the first few minutes of the Universe through fusion reactions is known as Big Bang nucleosynthesis (BBN). The principles that BBN is based upon are well understood from nuclear physics; these are principles that can be carefully tested in laboratories. Since the Universe was expanding and cooling rapidly, the BBN era did not last very long. It produced isotopes through lithium, but heavier elements came a few hundred million years later when the first stars created the conditions in which the heavy elements could form.
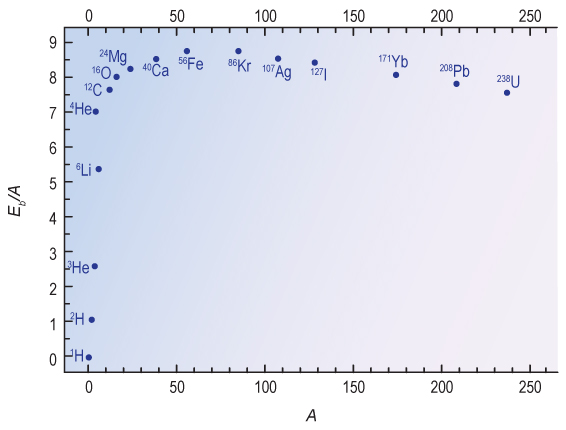
The relative abundance of elements depends on both the overall temperature and the weak nuclear force. Hi temperatures are needed to overcome the electric forces that repel protons from one another. The weak nuclear force is responsible for radioactive decay in unstable nuclei, and it affects the basic physics governing the protons and neutrons which must be combined to create heavier elements. Recall that protons and neutrons release energy, called binding energy (Figure 16.6), when combined to form nuclei during nuclear fusion. The overall peak of the binding energy curve occurs at iron, but at low atomic weights the most tightly bound nucleus is 4He. It is therefore favored for production compared to other light isotopes during BBN.
One limitation on fusion reactions in the early Universe is that a free neutron will decay into a proton (plus an electron and an antineutrino) even at low temperatures - this is a spontaneous reaction, a result of the weak nuclear force. On the other hand, a proton can only be turned into a neutron (plus a positron and electron neutrino) at high temperature and pressure. Energy input is needed for the reaction to run in that direction. For the first second in the history of the Universe (when the temperature is greater than 1010 K), protons and neutrons could change back and forth easily because there was ample energy to convert protons back to neutrons. After that, the temperature of the Universe dropped and there was not enough energy in collisions to turn protons into neutrons. The balance of these reactions, set by the mass difference between the neutron and proton, fixed the ratio of protons to neutrons at about 7:1. This ratio in turn dictated what elements could form.
Since normal hydrogen consists of one proton, and 4He consists of two protons and two neutrons, we can see from the ratio of neutrons to protons how we would get roughly the observed abundance of each of them. In the next two activities, you will determine these by mass and by number.
How do we go from a proton-to-neutron ratio of 7:1, to 75% of the atomic mass in the Universe being hydrogen and 25% being helium? We can count the number of atoms and then figure out the mass.
To do so, you will need the following information:
- To make a 1H nucleus, 1 proton is required.
- To make a 4He nucleus, 2 protons and 2 neutrons are required.
- When BBN started there were 7 protons for every 1 neutron.
Figure A.16.1 shows protons and neutrons in the correct proportions at the start of BBN as well as how they must be grouped to create 4He.

1.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
2.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
3.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
Each particle has a mass unit of 1 amu (atomic mass unit).
4.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
5. Take your answer to question 3 divided by question 1 to determine the hydrogen fraction by mass. What percentage is this?
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
In the previous activity, we worked out the mass fractions of hydrogen and helium in the Universe given a proton:neutron ratio of 7:1. Another way to discuss atomic abundances is in terms of numbers of atoms.
1.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
2.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
3.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
When quoting abundances in terms of number instead of mass, each atom, rather than each constituent particle, counts as 1.
4.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
5.
Login with LibreOne to view this question
NOTE: If you typically access ADAPT assignments through an LMS like Canvas, you should open this page there.
In the previous activities, you saw why the abundance is roughly three-fourths (75%) hydrogen and one-fourth (25%) helium by mass (that is, about 75% of the mass of atoms in the Universe is in hydrogen). If we count by number instead of mass, these percentages are 92% for hydrogen and 8% for helium. Scientists who study BBN use computer codes that include all of the possible reactions between neutrons, protons, and the light elements they produce. These codes give ranges for the yields of various elements at the end of the BBN era, and these yields must be compared to the observed abundance, as we will see.
Of course, elements besides helium were also produced. Isotopes such as deuterium were created by the collisions of protons and neutrons. At very high temperatures, additional collisions with other protons and neutrons, or especially with photons, tended to break deuterium apart. However, as the temperature dropped, collisions were no longer energetic enough to destroy deuterium. Deuterium atoms could then survive long enough to react with themselves and with protons and neutrons to make both 3He and 4He. Collisions could also form the radioactive isotope of hydrogen called tritium (3H or T). The first reactions that occurred are shown schematically in Figure 16.7.

In principle it is possible to build all the way up the curve of binding energy to iron, which has the highest binding energy of any nucleus. In fact, there was only time for the chain to build up to lithium (7Li), and possibly a tiny amount of beryllium (9Be). Protons have positive charge, and there are no negative charges in the nucleus, so it takes energy to get them to come together. Once they are very close, within about 10-15 m, the strong nuclear force can bind them together. Before that can happen they must be moving fast enough to approach positively charged nuclei containing one or several protons. This means that heavier elements require higher temperatures and more time in order to overcome the greater electric repulsion caused by their greater number of charges.
But the Universe was expanding, lowering both the temperature and the density. At around 3 or 4 minutes, when lithium was produced, the density and temperature had dropped too low to allow much of anything else to form. Therefore, only hydrogen, helium and lithium were available when the earliest stars formed several hundred million years later. As we have seen, almost all of this material was 1H and 4He. The reactions that could form the lightest elements beyond hydrogen and helium are shown in Figure 16.8. The formation of beryllium is speculative, as we do not have evidence that it was actually produced by BBN. What's more, any that was produced could well have been destroyed inside stars since then.

It was not time alone that weighed against building elements beyond lithium. As shown in Figure 16.9, a serious bottleneck results from that fact that all isotopes with mass numbers 5 and 8 are unstable. Even stars have difficulty getting past these gaps. However, what stars have that the early Big Bang did not is time. At a minimum, stars have millions of years to work with, and they manage to build heavier elements by combining three helium nuclei. That is a slow process, and there was no time for it in the first few minutes of the early Universe. Even given the extra time, the elements between helium and carbon are very rare, an indication of their fragility. Consider the abundance of each as plotted in Figure 16.3.

In this activity you will build atoms, simulating the process of Big Bang nucleosynthesis.
You will have 4 minutes to create as many atoms as you can, just as the Universe did! See how many you can make. As you do the activity, here are a few things to keep in mind:
- Protons (brown) and neutrons (white) will move across the screen for you to use as raw material.
- To combine any two particles, click on each of them once.
- You score more points for creating heavier elements.
- Watch out for photons-they are flying by and can break apart atoms, causing you to lose points.
- If particles are too far away, they will not combine with each other.
- If a nuclear reaction is forbidden, the particles will not combine.
- Unstable atoms will fall apart instantly.
Play Activity
In the previous activity, you probably saw that your ability to create elements depended on how many protons and neutrons were available and how many photons were breaking the elements apart. Detailed computations show that the abundance of deuterium, 3He, and 7Li are very sensitive to the density of baryons (particles made of three quarks - protons and neutrons for our purposes), from which they are made. The dependence is generally stated in terms of the baryon-to-photon ratio, η (pronounced “eta”)-the ratio of the number of baryons to the number of particles of light. A higher density of baryons (larger η) means reactions go faster because there are more particles around to combine. More photons, on the other hand (smaller value of η), means nuclei like deuterium are more easily photo-dissociated (split apart by the photons). That makes building heavier elements more difficult. The measured abundance of these elements provides a way to probe the actual value of η, and hence conditions in the early Universe.
The abundances relevant to determining η are those present in the early Universe, before stars began to modify them with their own nuclear fusion. The pattern of elemental abundance that was in place just after Big Bang nucleosynthesis ended is called the primordial abundance. It can be difficult to measure because stellar processes have modified this pattern over the intervening billions of years. For instance, deuterium is easily fused to form helium inside stars. So if we look at a young star like the Sun we will measure a lower deuterium abundance than was present in the early Universe. The solar deuterium abundance is even lower than it was in the material that formed the Sun. This is because stars destroy deuterium, they don’t create it.
Similar problems hamper the measurement of other light elements’ primordial abundance. However, by choosing to study very old stars we can minimize the effects of stellar evolution. Better still, if we look at diffuse clouds in intergalactic space, far away from any galaxies (and thus stars), we can measure the elemental abundance that should be truly primordial. That is what has been done for deuterium - we measure its absorption features in the absorption spectra of distant quasars (see Figure 16.10). The absorption features in these spectra are not due to the quasar itself, which just serves as a background light source. The absorption is caused by gas clouds located between us and the quasar, and most of these are in the space between galaxies. They are thus far away from any stars that would have polluted them with the products of stellar nuclear fusion.

Measurements of the primordial abundance of the light elements constrain the baryon fraction, that is, the fraction of normal matter in the Universe. A comparison of the measured abundance and the abundance computed from BBN has important implications for the viability of the Big Bang theory and the composition of the Universe. We can plot the abundance in terms of either baryon fraction or η, as in Figure 16.11. This figure is somewhat compact and requires a brief explanation to be understood. The vertical axis, which relays the fraction of each element, has a broken scale. This is done so that all these isotopes can be shown together, given that they cover a vast range of abundance. We will break each part down separately.
The top part of Figure 16.11 shows the mass fraction of 4He, just as we calculated above. Measurements place this value at between 24% and 25%, similar to our previous estimate. The abundance of 4He is not very sensitive to the baryon fraction, so it remains high for all of the values of baryon density on the plot. This is because the 4He nucleus is extremely stable. It has the highest binding energy of any light atom, as can be seen in Figure 16.6. As a result, most of the free neutrons at the beginning of the BBN era are combined with protons to form 4He. The faster the reactions run (the higher the baryon density) the faster this happens.
Below 4He is shown the abundance for deuterium, 3He, and 7Li. They are all shown in terms of number of atoms of each, per atom of hydrogen. So, for instance, deuterium is measured to be a few times 10-5 as abundant as hydrogen. That means that out of every fifty thousand or so hydrogen atoms, one will be deuterium. 3He is about half as abundant, though only upper limits are given; this is indicated by the downward-facing arrow. Finally, 7Li is measured to be a few times 10-10 (note the break in the vertical axis between 3He/D and 7Li), so for every 7Li atom in the early Universe there were about 5 billion hydrogen atoms.
Each of these measurements has uncertainty associated with it, and these are shown by the boxes on the plot; they indicate the range over which the measurements are able to constrain the abundance of each isotope. Because the abundances for each isotope are independently measured, the uncertainty boxes are not all the same size, and in fact for 3He there are only limits on the abundance: we know that it must be below about 10-5 of hydrogen.
The favored status of 4He is apparent in Figure 16.11, where we see that as its abundance goes up, the abundance of 3He and 2H (deuterium) go down. The other light isotopes are converted to 4He at higher baryon fractions because nuclear reactions can proceed more quickly, and it is this effect that causes the graphs to trend as they do.

Figure 16.11 shows a range of possible abundance for each element, both from theoretical calculations and from the measurements of each. The tightest limits are given by the abundance of deuterium; the other abundance measurements are consistent with the deuterium measurement. Taking them together we find the baryon fraction is 0.04 +/- 0.004. This value for the baryon density is consistent with the value we found using CMB measurements. It is several times larger than that found from luminous matter alone, indicating that there are many baryons that do not shine.
It is remarkable that the abundance of light atoms predicted by BBN are so well borne out by observations. The predictions for various isotopes range over 10 orders of magnitude, and yet all agree within the uncertainties of the measurements - which are considerably smaller. Deuterium in particular sets very stringent limits on the baryon density, and it is right in line with what is predicted by other isotopes. The strong agreement between these several isotopes is one of the lynch pins of modern cosmology. The agreement provides highly compelling evidence in favor of the standard hot Big Bang model of the early Universe.
In this chapter, and throughout much of the module, we have repeatedly stated that the Universe was denser and hotter when it was younger. Or equivalently, we have said that the Universe must have cooled as it expanded. Why must this be the case? If you have had a course in chemistry or physics you know the answer, but if not (or maybe even if you did) you might find these statements confusing. We will now explain why gases exhibit this property.
Consider a gas inside a closed container from which it cannot leak out. If you imagine that the container is a cylinder with one end free to move, then you could push or pull on the cylinder to change the volume occupied by the gas, and thus its density. A hand bicycle pump is an example of this sort of device. What effect would this have on the gas?
From experience you probably know that you must push on such a cylinder in order to compress it, and the more you manage to squeeze the gas, the harder it becomes to compress it further, sort of like trying to squeeze a tube of toothpaste with the cap on. The reason for the increasing difficulty is two-fold.
The first cause of your increasing effort is that you are expending energy to compress the gas. That energy is not lost: energy is always conserved. The only place for the energy to go in this case is into the gas. The energy you expend pushes on the gas particles and imparts a higher kinetic energy into them, so they move faster because you have sped them up. Since the particles are moving faster, they strike the walls of the container with a greater force. That increases the pressure on the walls and is the first reason you must push harder.
The second reason you have to exert more effort to compress a canister of gas is because the gas volume is getting smaller as you push inward. That means that the particles do not have to travel as far when they bounce between one wall and the opposite wall; they strike each wall more often. Because the particles are moving faster, they travel that shorter distance in a shorter time than would would be true if they moved at a lower speed. This also causes the particles to hit the walls more frequently.
The net result is that by compressing the gas you speed up the particles and cause them to hit the walls more often and with greater force. The fact that the gas particles are moving faster is generally conveyed by saying their temperature (T) has increased. That they are confined to a smaller space means that their volume (V) has decreased, and these changes have led the pressure (P) to increase. The total number of particles (N) in this case has remained the same because no gas has leaked out of the cylinder. We could also think about what happens in terms of the number density, N / V, which is increasing in this case.
The pressure, volume, number of particles, and temperature of a gas are related by the following equation.
PV=NkT
The lowercase k is called Boltzmann’s constant, which we have already seen. The equation is known as the ideal gas law. You might be familiar with this equation from a chemistry course. It is often written as below.
PV=nRT
Now, n is the number of moles and R is the ideal gas constant. Chemists like to use moles, physicists use the number of particles instead, but they are essentially the same. According to the ideal gas law, as the density or temperature of an ideal gas goes up, the pressure also tends to go up. Don't let the word "ideal" confuse you. Most gases follow this law quite closely. It fails to work when the particles interact strongly with one another, but at that point you are probably no longer dealing with a gas.
Conversely, if we let the volume of our cylinder expand, the gas inside would have to push against an outside pressure, expending energy to do so. The energy would have to come from the internal energy of the gas itself, thus lowering its temperature, density and pressure.
That is why gases cool as they expand and heat up when they are compressed. It is the basis of technologies like refrigerators and air conditioners. In the case of the Universe, it expands not against an external pressure, but against its own self-gravity. The result is the same: the gas within the Universe cools as a result of the expansion.


