14.7: Radiation
- Page ID
- 1592
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
By the end of this section, you will be able to:
- Discuss heat transfer by radiation.
- Explain the power of different materials.
You can feel the heat transfer from a fire and from the Sun. Similarly, you can sometimes tell that the oven is hot without touching its door or looking inside—it may just warm you as you walk by. The space between the Earth and the Sun is largely empty, without any possibility of heat transfer by convection or conduction. In these examples, heat is transferred by radiation. That is, the hot body emits electromagnetic waves that are absorbed by our skin: no medium is required for electromagnetic waves to propagate. Different names are used for electromagnetic waves of different wavelengths: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.

The energy of electromagnetic radiation depends on the wavelength (color) and varies over a wide range: a smaller wavelength (or higher frequency) corresponds to a higher energy. Because more heat is radiated at higher temperatures, a temperature change is accompanied by a color change. Take, for example, an electrical element on a stove, which glows from red to orange, while the higher-temperature steel in a blast furnace glows from yellow to white. The radiation you feel is mostly infrared, which corresponds to a lower temperature than that of the electrical element and the steel. The radiated energy depends on its intensity, which is represented in the figure below by the height of the distribution.
Electromagnetic Waves explains more about the electromagnetic spectrum and Introduction to Quantum Physics discusses how the decrease in wavelength corresponds to an increase in energy.
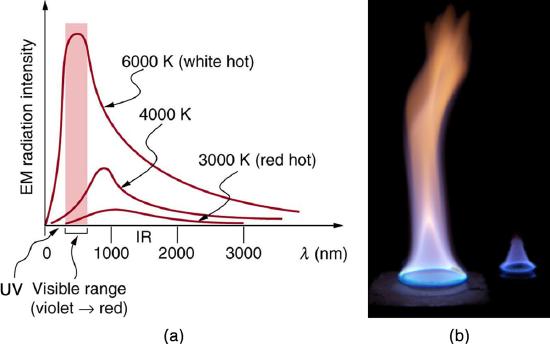
All objects absorb and emit electromagnetic radiation. The rate of heat transfer by radiation is largely determined by the color of the object. Black is the most effective, and white is the least effective. People living in hot climates generally avoid wearing black clothing, for instance. Similarly, black asphalt in a parking lot will be hotter than adjacent gray sidewalk on a summer day, because black absorbs better than gray. The reverse is also true—black radiates better than gray. Thus, on a clear summer night, the asphalt will be colder than the gray sidewalk, because black radiates the energy more rapidly than gray. An ideal radiator is the same color as an ideal absorber, and captures all the radiation that falls on it. In contrast, white is a poor absorber and is also a poor radiator. A white object reflects all radiation, like a mirror. (A perfect, polished white surface is mirror-like in appearance, and a crushed mirror looks white.)

Gray objects have a uniform ability to absorb all parts of the electromagnetic spectrum. Colored objects behave in similar but more complex ways, which gives them a particular color in the visible range and may make them special in other ranges of the nonvisible spectrum. Take, for example, the strong absorption of infrared radiation by the skin, which allows us to be very sensitive to it.
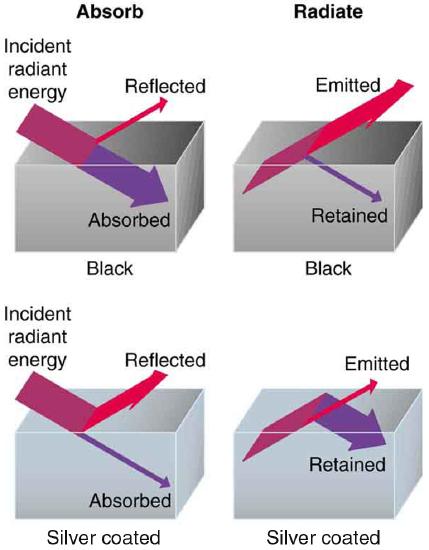
The rate of heat transfer by emitted radiation is determined by the Stefan-Boltzmann law of radiation:
\[\dfrac{Q}{t} = \sigma e AT^4,\]
where \(\sigma = 5.67 \times 10^{-8} \, J/s \cdot m^2 \cdot k^4\) is the Stefan-Boltzmann constant, \(A\) is the surface area of the object, and \(T\) is its absolute temperature in kelvin. The symbol \(e\) stands for the emissivity of the object, which is a measure of how well it radiates. An ideal jet-black (or black body) radiator has \(e = 1\), whereas a perfect reflector has \(e = 0\). Real objects fall between these two values. Take, for example, tungsten light bulb filaments which have an \(e\) of about 0.5, and carbon black (a material used in printer toner), which has the (greatest known) emissivity of about 0.99.
The radiation rate is directly proportional to the fourth power of the absolute temperature—a remarkably strong temperature dependence. Furthermore, the radiated heat is proportional to the surface area of the object. If you knock apart the coals of a fire, there is a noticeable increase in radiation due to an increase in radiating surface area.

Skin is a remarkably good absorber and emitter of infrared radiation, having an emissivity of 0.97 in the infrared spectrum. Thus, we are all nearly (jet) black in the infrared, in spite of the obvious variations in skin color. This high infrared emissivity is why we can so easily feel radiation on our skin. It is also the basis for the use of night scopes used by law enforcement and the military to detect human beings. Even small temperature variations can be detected because of the \(T^4\) dependence. Images, called thermographs, can be used medically to detect regions of abnormally high temperature in the body, perhaps indicative of disease. Similar techniques can be used to detect heat leaks in homes Figure \(\PageIndex{5}\), optimize performance of blast furnaces, improve comfort levels in work environments, and even remotely map the Earth’s temperature profile.
All objects emit and absorb radiation. The net rate of heat transfer by radiation (absorption minus emission) is related to both the temperature of the object and the temperature of its surroundings. Assuming that an object with a temperature \(T_1\) is surrounded by an environment with uniform temperature \(T_2\) the net rate of heat transfer by radiation is \[\dfrac{Q_{net}}{t} = \sigma e A(T_2^4 - T_1^4),\] where \(e\) is the emissivity of the object alone. In other words, it does not matter whether the surroundings are white, gray, or black; the balance of radiation into and out of the object depends on how well it emits and absorbs radiation. When \(T_2 > T_1\), the quantity \(Q_{net}/t\) is positive; that is, the net heat transfer is from hot to cold.
Take-Home Experiment: Temperature in the Sun
Place a thermometer out in the sunshine and shield it from direct sunlight using an aluminum foil. What is the reading? Now remove the shield, and note what the thermometer reads. Take a handkerchief soaked in nail polish remover, wrap it around the thermometer and place it in the sunshine. What does the thermometer read?
Example \(\PageIndex{1}\): Calculate the Net Heat Transfer of a Person: Heat Transfer by Radiation
What is the rate of heat transfer by radiation, with an unclothed person standing in a dark room whose ambient temperature is \(22.0^oC\). The person has a normal skin temperature of \(33.0^oC\) and a surface area of \(1.50 \, m^2\). The emissivity of skin is 0.97 in the infrared, where the radiation takes place.
Strategy
We can solve this by using the equation for the rate of radiative heat transfer.
Solution
Insert the temperatures values \(T_2 = 295 \, K\) and \(T_1 = 306 \, K\), so that \[\dfrac{Q}{t} = \sigma e A(T_2^4 - T_1^4)\] \[= (5.67 \times 10^{-8} \, J/s \cdot m^2 \cdot k^4)(0.97)(1.50 \, m^2) [(295 \, K)^4 - (306 \, K)^4]\] \[= -99 \, J/s = -99 \, W.\]
Discussion
This value is a significant rate of heat transfer to the environment (note the minus sign), considering that a person at rest may produce energy at the rate of 125 W and that conduction and convection will also be transferring energy to the environment. Indeed, we would probably expect this person to feel cold. Clothing significantly reduces heat transfer to the environment by many methods, because clothing slows down both conduction and convection, and has a lower emissivity (especially if it is white) than skin.
The Earth receives almost all its energy from radiation of the Sun and reflects some of it back into outer space. Because the Sun is hotter than the Earth, the net energy flux is from the Sun to the Earth. However, the rate of energy transfer is less than the equation for the radiative heat transfer would predict because the Sun does not fill the sky. The average emissivity (e) of the Earth is about 0.65, but the calculation of this value is complicated by the fact that the highly reflective cloud coverage varies greatly from day to day. There is a negative feedback (one in which a change produces an effect that opposes that change) between clouds and heat transfer; greater temperatures evaporate more water to form more clouds, which reflect more radiation back into space, reducing the temperature. The often mentioned greenhouse effect is directly related to the variation of the Earth’s emissivity with radiation type (see the figure given below). The greenhouse effect is a natural phenomenon responsible for providing temperatures suitable for life on Earth. The Earth’s relatively constant temperature is a result of the energy balance between the incoming solar radiation and the energy radiated from the Earth. Most of the infrared radiation emitted from the Earth is absorbed by carbon dioxide \((CO_2)\) and water \((H_2O)\) in the atmosphere and then re-radiated back to the Earth or into outer space. Re-radiation back to the Earth maintains its surface temperature about \(40^oC\) higher than it would be if there was no atmosphere, similar to the way glass increases temperatures in a greenhouse.
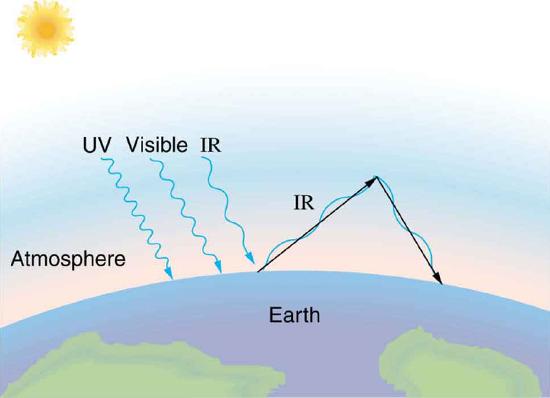
The greenhouse effect is also central to the discussion of global warming due to emission of carbon dioxide and methane (and other so-called greenhouse gases) into the Earth’s atmosphere from industrial production and farming. Changes in global climate could lead to more intense storms, precipitation changes (affecting agriculture), reduction in rain forest biodiversity, and rising sea levels.
Heating and cooling are often significant contributors to energy use in individual homes. Current research efforts into developing environmentally friendly homes quite often focus on reducing conventional heating and cooling through better building materials, strategically positioning windows to optimize radiation gain from the Sun, and opening spaces to allow convection. It is possible to build a zero-energy house that allows for comfortable living in most parts of the United States with hot and humid summers and cold winters.

Conversely, dark space is very cold, about \(3K (-454^oF)\), so that the Earth radiates energy into the dark sky. Owing to the fact that clouds have lower emissivity than either oceans or land masses, they reflect some of the radiation back to the surface, greatly reducing heat transfer into dark space, just as they greatly reduce heat transfer into the atmosphere during the day. The rate of heat transfer from soil and grasses can be so rapid that frost may occur on clear summer evenings, even in warm latitudes.
Exercise \(\PageIndex{1}\)
What is the change in the rate of the radiated heat by a body at the temperature \(T_1 = 20^oC\) compared to when the body is at the temperature \(T_2 = 40^oC\)?
- Answer
-
The radiated heat is proportional to the fourth power of the absolute temperature. Because \(T_1 = 293 \, K\) and \(T_2 = 313 \, K\), the rate of heat transfer increases by about 30 percent of the original rate.
Career Connection: Energy Conservation Consultation
The cost of energy is generally believed to remain very high for the foreseeable future. Thus, passive control of heat loss in both commercial and domestic housing will become increasingly important. Energy consultants measure and analyze the flow of energy into and out of houses and ensure that a healthy exchange of air is maintained inside the house. The job prospects for an energy consultant are strong.
Problem Solving Strategies for the Methods of Heat Transfer
- Examine the situation to determine what type of heat transfer is involved.
- Identify the type(s) of heat transfer—conduction, convection, or radiation.
- Identify exactly what needs to be determined in the problem (identify the unknowns). A written list is very useful.
- Make a list of what is given or can be inferred from the problem as stated (identify the knowns).
- Solve the appropriate equation for the quantity to be determined (the unknown).
- For conduction, equation \(\dfrac{Q}{t} = \dfrac{kA(T_2 - T_1)}{d} \) is appropriate. [link] lists thermal conductivities. For convection, determine the amount of matter moved and use equation \(Q = mc\Delta T\), to calculate the heat transfer involved in the temperature change of the fluid. If a phase change accompanies convection, equation \(Q = mL_f\) or \(Q = mL_v\) is appropriate to find the heat transfer involved in the phase change. [link] lists information relevant to phase change. For radiation, equation \(\dfrac{Q_{net}}{t} = \sigma e A (T_2^4 - T_1^4)\) gives the net heat transfer rate.
- Insert the knowns along with their units into the appropriate equation and obtain numerical solutions complete with units.
- Check the answer to see if it is reasonable. Does it make sense?
Summary
- Radiation is the rate of heat transfer through the emission or absorption of electromagnetic waves.
- The rate of heat transfer depends on the surface area and the fourth power of the absolute temperature: \[\dfrac{Q}{t} = \sigma eAT^4,\] where \(\sigma = 5.67 \times 10^{-8} \, J/s \cdot m^2 \cdot K^4\) is the Stefan-Boltzmann constant and \(e\) is the emissivity of the body. For a black body, \(e = 1\) whereas a shiny white or perfect reflector has \(e = 0\), with real objects having values of e between 1 and 0. The net rate of heat transfer by radiation is \[\dfrac{Q_{net}}{t} = \sigma eA(T_2^4 - T_1^4)\] where \(T_1\) is the temperature of an object surrounded by an environment with uniform temperature \(T_2\) and \(e\) is the emissivity of the object.
Glossary
- emissivity
- measure of how well an object radiates
- greenhouse effect
- warming of the Earth that is due to gases such as carbon dioxide and methane that absorb infrared radiation from the Earth’s surface and reradiate it in all directions, thus sending a fraction of it back toward the surface of the Earth
- net rate of heat transfer by radiation
- is \(\frac{Q_{net}}{t}=σeA(T^4_2−T^4_1)\)
- radiation
- energy transferred by electromagnetic waves directly as a result of a temperature difference
- Stefan-Boltzmann law of radiation
- \(\frac{Q}{t}=σeAT^4\), where σ is the Stefan-Boltzmann constant, \(A\) is the surface area of the object, \(T\) is the absolute temperature, and \(e\) is the emissivity


