6.1: Pressure and Temperature
- Page ID
- 951
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)When we heat an object, we speed up the mind-bogglingly complex random motion of its molecules. One method for taming complexity is the conservation laws, since they tell us that certain things must remain constant regardless of what process is going on. Indeed, the law of conservation of energy is also known as the first law of thermodynamics.
But as alluded to in the introduction to this chapter, conservation of energy by itself is not powerful enough to explain certain empirical facts about heat. A second way to sidestep the complexity of heat is to ignore heat's atomic nature and concentrate on quantities like temperature and pressure that tell us about a system's properties as a whole. This approach is called macroscopic in contrast to the microscopic method of attack. Pressure and temperature were fairly well understood in the age of Newton and Galileo, hundreds of years before there was any firm evidence that atoms and molecules even existed.
Unlike the conserved quantities such as mass, energy, momentum, and angular momentum, neither pressure nor temperature is additive. Two cups of coffee have twice the heat energy of a single cup, but they do not have twice the temperature. Likewise, the painful pressure on your eardrums at the bottom of a pool is not affected if you insert or remove a partition between the two halves of the pool.
We restrict ourselves to a discussion of pressure in fluids at rest and in equilibrium. In physics, the term “fluid” is used to mean either a gas or a liquid. The important feature of a fluid can be demonstrated by comparing with a cube of jello on a plate. The jello is a solid. If you shake the plate from side to side, the jello will respond by shearing, i.e., by slanting its sides, but it will tend to spring back into its original shape. A solid can sustain shear forces, but a fluid cannot. A fluid does not resist a change in shape unless it involves a change in volume.
5.1.1 Pressure
If you're at the bottom of a pool, you can't relieve the pain in your ears by turning your head. The water's force on your eardrum is always the same, and is always perpendicular to the surface where the eardrum contacts the water. If your ear is on the east side of your head, the water's force is to the west. If you keep your ear in the same spot while turning around so your ear is on the north, the force will still be the same in magnitude, and it will change its direction so that it is still perpendicular to the eardrum: south. This shows that pressure has no direction in space, i.e., it is a scalar. The direction of the force is determined by the orientation of the surface on which the pressure acts, not by the pressure itself. A fluid flowing over a surface can also exert frictional forces, which are parallel to the surface, but the present discussion is restricted to fluids at rest.
Experiments also show that a fluid's force on a surface is proportional to the surface area. The vast force of the water behind a dam, for example, in proportion to the dam's great surface area. (The bottom of the dam experiences a higher proportion of its force.)
Based on these experimental results, it appears that the useful way to define pressure is as follows. The pressure of a fluid at a given point is defined as \(F_\perp/A\), where \(A\) is the area of a small surface inserted in the fluid at that point, and \(F_\perp\) is the component of the fluid's force on the surface which is perpendicular to the surface. (In the case of a moving fluid, fluid friction forces can act parallel to the surface, but we're only dealing with stationary fluids, so there is only an \(F_\perp\).)
This is essentially how a pressure gauge works. The reason that the surface must be small is so that there will not be any significant difference in pressure between one part of it and another part. The SI units of pressure are evidently \(\text{N}/\text{m}^2\), and this combination can be abbreviated as the pascal, 1 Pa=1 \(\text{N}/\text{m}^2\). The pascal turns out to be an inconveniently small unit, so car tires, for example, normally have pressures imprinted on them in units of kilopascals.
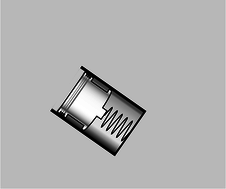
a / A simple pressure gauge consists of a cylinder open at one end, with a piston and a spring inside. The depth to which the spring is depressed is a measure of the pressure. To determine the absolute pressure, the air needs to be pumped out of the interior of the gauge, so that there is no air pressure acting outward on the piston. In many practical gauges, the back of the piston is open to the atmosphere, so the pressure the gauge registers equals the pressure of the fluid minus the pressure of the atmosphere.
| Example 1: Pressure in U.S. units |
|---|
|
In U.S. units, the unit of force is the pound, and the unit of distance is the inch. The unit of pressure is therefore pounds per square inch, or p.s.i. (Note that the pound is not a unit of mass.) |
| Example 2: Atmospheric pressure in U.S. and metric units |
|---|
|
\(\triangleright\) A figure that many people in the U.S. remember is that atmospheric pressure is about 15 pounds per square inch. What is this in metric units? \(\triangleright\) \[\begin{align*} (\text{15 lb})/(\text{1 in}^2) &= \frac{68\ \text{N}}{(0.0254\ \text{m})^2}\\ &= 1.0\times10^5\ \text{N}/\text{m}^2 \\ &= 100\ \text{kPa} \end{align*}\] |
Benjamin Crowell (Fullerton College). Conceptual Physics is copyrighted with a CC-BY-SA license.


