8.3: The Ideal Gas Law
- Page ID
- 46201
Learning Objectives
- State and explain the ideal gas law using Boltzmann's constant
- Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules in a given volume.

In this section, we continue to explore the thermal behavior of gases. In particular, we examine the characteristics of atoms and molecules that compose gases. (Most gases, for example nitrogen, \(\mathrm{N}_{2}\), and oxygen, \(\mathrm{O}_{2}\), are composed of two or more atoms. We will primarily use the term “molecule” in discussing a gas because the term can also be applied to monatomic gases, such as helium.)
Gases are easily compressed. Gases expand and contract very rapidly with temperature changes. In addition, you will note that most gases expand at the same rate, or have the same \(\beta\). This raises the question as to why gases should all act in nearly the same way, when liquids and solids have widely varying expansion rates.
The answer lies in the large separation of atoms and molecules in gases, compared to their sizes, as illustrated in Figure \(\PageIndex{2}\). Because atoms and molecules have large separations, forces between them can be ignored, except when they collide with each other during collisions. The motion of atoms and molecules (at temperatures well above the boiling temperature) is fast, such that the gas occupies all of the accessible volume and the expansion of gases is rapid. In contrast, in liquids and solids, atoms and molecules are closer together and are quite sensitive to the forces between them.
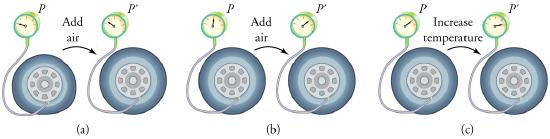
To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into an initially deflated tire. The tire’s volume first increases in direct proportion to the amount of air injected, without much increase in the tire pressure. Once the tire has expanded to nearly its full size, the walls limit volume expansion. If we continue to pump air into it, the pressure increases. The pressure will further increase when the car is driven and the tires move. Most manufacturers specify optimal tire pressure for cold tires. (See Figure \(\PageIndex{3}\).)
At room temperatures, collisions between atoms and molecules can be ignored. In this case, the gas is called an ideal gas, in which case the relationship between the pressure, volume, and temperature is given by the equation of state called the ideal gas law.
IDEAL GAS LAW
The ideal gas law states that
\[P V=N k T, \nonumber\]
where \(P\) is the absolute pressure of a gas, \(V\) is the volume it occupies, \(N\) is the number of atoms and molecules in the gas, and \(T\) is its absolute temperature. The constant \(k\) is called the Boltzmann constant in honor of Austrian physicist Ludwig Boltzmann (1844–1906) and has the value
\[k=1.38 \times 10^{-23} \mathrm{~J} / \mathrm{K}. \nonumber \]
The ideal gas law can be derived from basic principles, but was originally deduced from experimental measurements of Charles’ law (that volume occupied by a gas is proportional to temperature at a fixed pressure) and from Boyle’s law (that for a fixed temperature, the product \(PV\) is a constant). In the ideal gas model, the volume occupied by its atoms and molecules is a negligible fraction of \(V\). The ideal gas law describes the behavior of real gases under most conditions. (Note, for example, that \(N\) is the total number of atoms and molecules, independent of the type of gas.)
Let us see how the ideal gas law is consistent with the behavior of filling the tire when it is pumped slowly and the temperature is constant. At first, the pressure \(P\) is essentially equal to atmospheric pressure, and the volume \(V\) increases in direct proportion to the number of atoms and molecules \(N\) put into the tire. Once the volume of the tire is constant, the equation \(P V=N k T\) predicts that the pressure should increase in proportion to the number N of atoms and molecules.
Example \(\PageIndex{1}\): Calculating Pressure Changes Due to Temperature Changes: Tire Pressure
Suppose your bicycle tire is fully inflated, with an absolute pressure of \(7.00 \times 10^{5} \mathrm{~Pa}\) (a gauge pressure of just under \(90.0 \ \mathrm{lb} / \mathrm{in}^{2}\)) at a temperature of \(18.0^{\circ} \mathrm{C}\). What is the pressure after its temperature has risen to 35.0ºC? Assume that there are no appreciable leaks or changes in volume.
Strategy
The pressure in the tire is changing only because of changes in temperature. First we need to identify what we know and what we want to know, and then identify an equation to solve for the unknown.
We know the initial pressure \(P_{0}=7.00 \times 10^{5} \mathrm{~Pa}\), the initial temperature \(T_{0}=18.0^{\circ} \mathrm{C}\), and the final temperature \(T_{\mathrm{f}}=35.0^{\circ} \mathrm{C}\). We must find the final pressure \(P_{\mathrm{f}} \). How can we use the equation \(P V=N k T\)? At first, it may seem that not enough information is given, because the volume V and number of atoms N are not specified. What we can do is use the equation twice: \(P_{0} V_{0}=N k T_{0}\) and \(P_{\mathrm{f}} V_{\mathrm{f}}=N k T_{\mathrm{f}}\). If we divide \(P_{\mathrm{f}} V_{\mathrm{f}}\) by \(P_{0} V_{0}\) we can come up with an equation that allows us to solve for Pf.
\[\frac{P_{\mathrm{f}} V_{\mathrm{f}}}{P_{0} V_{0}}=\frac{N_{\mathrm{f}} k T_{\mathrm{f}}}{N_{0} k T_{0}} \nonumber\]
Since the volume is constant, \(V_{f}\) and \(V_{0}\) are the same and they cancel out. The same is true for \(N_{\mathrm{f}}\) and \(N_{\mathrm{0}}\), and \(k\), which is a constant. Therefore,
\[\frac{P_{\mathrm{f}}}{P_{0}}=\frac{T_{\mathrm{f}}}{T_{0}}. \nonumber\]
We can then rearrange this to solve for \(P_{f}\):
\[P_{\mathrm{f}}=P_{0} \frac{T_{\mathrm{f}}}{T_{0}}, \nonumber\]
where the temperature must be in units of kelvins, because \(T_{0}\) and \(T_{f}\) are absolute temperatures.
Solution
1. Convert temperatures from Celsius to Kelvin.
\[\begin{aligned}
&T_{0}=(18.0+273) \mathrm{K}=291 \mathrm{~K} \\
&T_{\mathrm{f}}=(35.0+273) \mathrm{K}=308 \mathrm{~K}
\end{aligned} \nonumber\]
2. Substitute the known values into the equation.
\[P_{\mathrm{f}}=P_{0} \frac{T_{\mathrm{f}}}{T_{0}}=7.00 \times 10^{5} \mathrm{~Pa}\left(\frac{308 \mathrm{~K}}{291 \mathrm{~K}}\right)=7.41 \times 10^{5} \mathrm{~Pa} \nonumber\]
Discussion
The final temperature is about 6% greater than the original temperature, so the final pressure is about 6% greater as well. Note that absolute pressure and absolute temperature must be used in the ideal gas law.
MAKING CONNECTIONS: TAKE-HOME EXPERIMENT—REFRIGERATING A BALLOON
Inflate a balloon at room temperature. Leave the inflated balloon in the refrigerator overnight. What happens to the balloon, and why?
Example \(\PageIndex{2}\): Calculating the Number of Molecules in a Cubic Meter of Gas
How many molecules are in a typical object, such as gas in a tire or water in a drink? We can use the ideal gas law to give us an idea of how large \(N\) typically is.
Calculate the number of molecules in a cubic meter of gas at standard temperature and pressure (STP), which is defined to be 0ºC and atmospheric pressure.
Strategy
Because pressure, volume, and temperature are all specified, we can use the ideal gas law \(P V=N k T\), to find \(N\).
Solution
1. Identify the knowns.
\[\begin{aligned}
T &=0^{\circ} \mathrm{C}=273 \mathrm{~K} \\
P &=1.01 \times 10^{5} \mathrm{~Pa} \\
V &=1.00 \mathrm{~m}^{3} \\
k &=1.38 \times 10^{-23} \mathrm{~J} / \mathrm{K}
\end{aligned} \nonumber\]
2. Identify the unknown: number of molecules, \(N\).
3. Rearrange the ideal gas law to solve for \(N\).
\[\begin{gathered}
P V=N k T \\
N=\frac{P V}{k T}
\end{gathered} \nonumber\]
4. Substitute the known values into the equation and solve for \(N\).
\[N=\frac{P V}{k T}=\frac{\left(1.01 \times 10^{5} \mathrm{~Pa}\right)\left(1.00 \mathrm{~m}^{3}\right)}{\left(1.38 \times 10^{-23} \mathrm{~J} / \mathrm{K}\right)(273 \mathrm{~K})}=2.68 \times 10^{25} \text { molecules } \nonumber\]
Discussion
The calculated number, \(2.68 \times 10^{25}\), is certainly very large. You might say that the volume of a cubic meter is also large (\(1 \mathrm{~m}^{3}=1000\)), but even in a small volume of \(1 \mathrm{~cm}^{3}\), which is about size of a thimble (\(1 \mathrm{~cm}^{3}=10^{-6} \mathrm{~m}^{3}\)), a gas at STP has \(2.68 \times 10^{19}\) molecules in it (still a very large number). Once again, note that \(N\) is the same for all types or mixtures of gases.
Section Summary
- The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas.
- The ideal gas law can be written in terms of the number of molecules of gas:
\[P V=N k T, \nonumber\]
where \(P\) is pressure, \(V\) is volume, \(T\) is temperature, \(N\) is number of molecules, and \(k\) is the Boltzmann constant\[k=1.38 \times 10^{-23} \mathrm{~J} / \mathrm{K}. \nonumber\]
- The ideal gas law is generally valid at temperatures well above the boiling temperature.
Glossary
- ideal gas law
- the physical law that relates the pressure and volume of a gas to the number of gas molecules or number of moles of gas and the temperature of the gas
- Boltzmann constant
- k , a physical constant that relates energy to temperature; k=1.38×10–23 J/K


