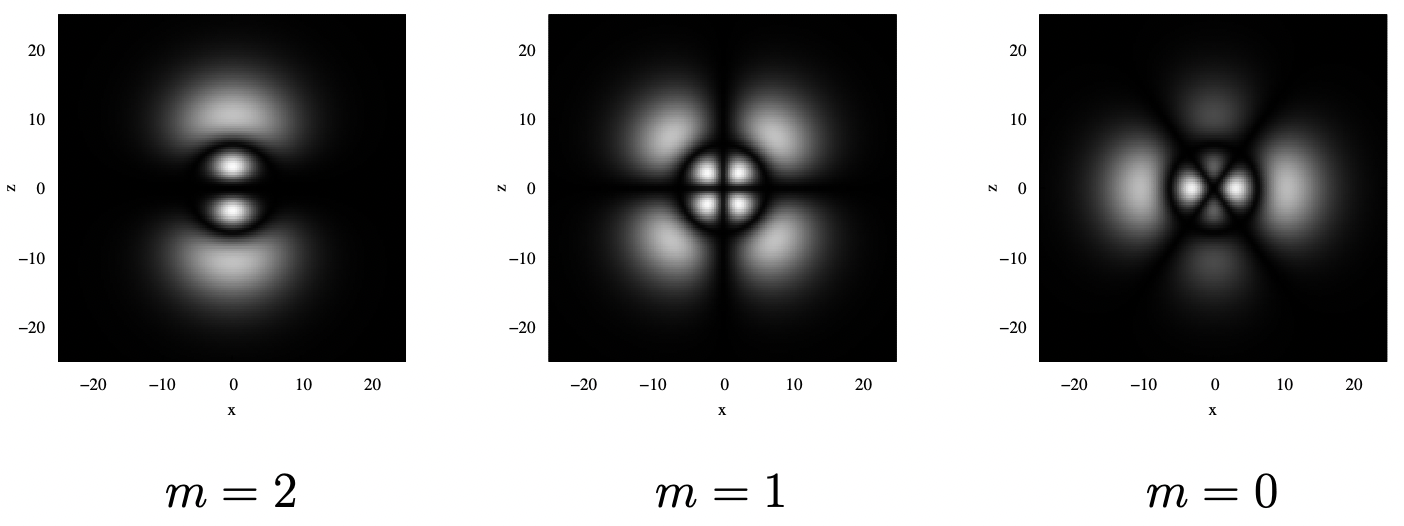
14.3.3: d Orbitals
( \newcommand{\kernel}{\mathrm{null}\,}\)
When l=2, the orbitals that are the solutions of the Hydrogen atom Schrödinger equation are called d orbitals. These orbitals only exist for shells with n=3 and greater, again because l must be less than n. As we saw with the p orbitals, the probability density for the electron in space is the same for +m and −m. As such, we’ll only plot the positive-m versions of the orbitals. As before, in addition to a 3d plot showing “shells” at a constant probability level, there is a 2d plot showing a cut in the x−z plane.
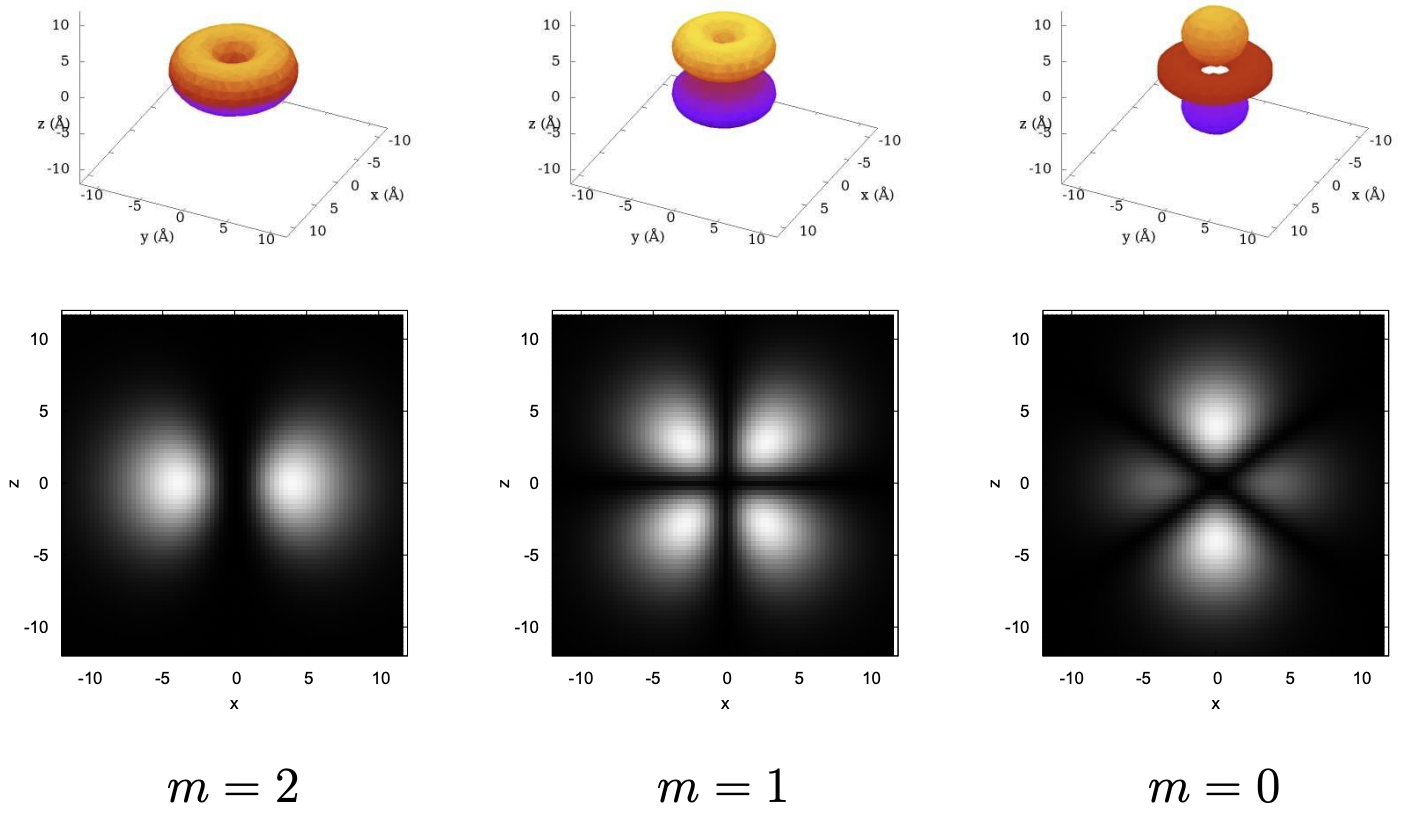
As with the p orbitals, as we go to the d orbitals in higher shells they get more interesting. Plotted below are the cuts through the x−z plane for the 4d orbitals: