1.4: The Uncertainty Principle
- Page ID
- 1646
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Waves are Fuzzy
As we have shown for wavepackets, the wave nature of particles implies that we cannot know both position and momentum of a particle to an arbitrary degree of accuracy—if \(\Delta x\) represents the uncertainty in our knowledge of position, and \(\Delta p\) that of momentum, then
\[ \Delta p \Delta x \sim h \tag{1.4.1} \]
where \(h\) is Planck’s constant. In the real world, particles are three-dimensional and we should say \[ \Delta p_x \Delta x \sim h \tag{1.4.2} \]
with corresponding equations for the other two spatial directions. The fuzziness about position is related to that of momentum in the same direction.
Let’s see how this works by trying to measure y-position and y-momentum very accurately. Suppose we have a source of electrons, say, an electron gun in a CRT (cathode ray tube, such as an old-fashioned monitor). The beam spreads out a bit, but if we interpose a sheet of metal with a slit of width \(w\), then for particles that make it through the slit, we know \(y\) with an uncertainty \(\Delta y=w\). Now, if the slit is a long way downstream from the electron gun source, we also know \(p_y\) very accurately as the electron reaches the slit, because to make it to the slit the electron’s velocity would have to be aimed just right.
But does the measurement of the electron’s y position—in other words, having it go through the slit—affect its y momentum? The answer is yes. If it didn’t, then sending a stream of particles through the slit they would all hit very close to the same point on a screen placed further downstream. But we know from experiment that this is not what happens—a single slit diffraction pattern builds up, of angular width \(\theta \sim \lambda/w\), where the electron’s de Broglie wavelength \(\lambda\) is given by \(p_x \cong h/\lambda\) (there is a negligible contribution to \(\lambda\) from the y-momentum). The consequent uncertainty in \(p_y\) is
\[ \Delta p_y/p_x \sim \theta \sim \lambda/w \tag{1.4.3} \]
Putting in \(p_x = h/\lambda\), we find immediately that
\[ \Delta p_y \sim h/w \tag{1.4.4} \]
so the act of measuring the electron’s y position has fuzzed out its y momentum by precisely the amount required by the uncertainty principle.
Trying to Beat the Uncertainty Principle
In order to understand the Uncertainty Principle better, let’s try to see what goes wrong when we actually try to measure position and momentum more accurately than allowed.
For example, suppose we look at an electron through a microscope. What could we expect to see? Of course, you know that if we try to look at something really small through a microscope it gets blurry—a small sharp object gets diffraction patterns around its edges, indicating that we are looking at something of size comparable to the wavelength of the light being used. If we look at something much smaller than the wavelength of light—like the electron—we would expect a diffraction pattern of concentric rings with a circular blob in the middle. The size of the pattern is of order the wavelength of the light, in fact from optics it can be shown to be \(\sim\lambda f/d\) where \(d\) is the diameter of the object lens of the microscope, \(f\) the focal distance (the distance from the lens to the object). We shall take \(f /d \sim 1\), as it usually is. So looking at an object the size of an electron should give a diffraction pattern centered on the location of the object. That would seem to pin down its position fairly precisely.
What about the momentum of the electron? Here a problem arises that doesn’t matter for larger objects—the light we see has, of course, bounced off the electron, and so the electron has some recoil momentum. That is, by bouncing light off the electron we have given it some momentum. Can we say how much? To make it simple, suppose we have good eyes and only need to bounce one photon off the electron to see it. We know the initial momentum of the photon (because we know the direction of the light beam we’re using to illuminate the electron) and we know that after bouncing off, the photon hits the object lens and goes through the microscope, but we don’t know where the photon hit the object lens. The whole point of a microscope is that all the light from a point, light that hits the object lens in different places, is all focused back to one spot, forming the image (apart from the blurriness mentioned above). So if the light has wavelength \(\lambda\), its constituent photons have momentum \(\sim h/\lambda\), and from our ignorance of where the photon entered the microscope we are uncertain of its x-direction momentum by an amount \(\sim h/\lambda\). Necessarily, then, we have the same uncertainty about the electron’s x-direction momentum, since this was imparted by the photon bouncing off.
But now we have a problem. In our attempts to minimize the uncertainty in the electron’s momentum, by only using one photon to detect it, we are not going to see much of the diffraction pattern discussed above—such diffraction patterns are generated by many photons hitting the film, retina or whatever detecting equipment is being used. A single photon generates a single point (at best!). This point will most likely be within of order \(\lambda\) of the center of the pattern, but this leaves us with an uncertainty in position of order \(\lambda\).
Therefore, in attempting to observe the position and momentum of a single electron using a single photon, we find an uncertainty in position \(\Delta x\sim\lambda\), and in momentum \(\Delta p_x\sim h/\lambda\). These results are in accordance with Heisenberg’s Uncertainty Principle \(\Delta x\Delta p_x\sim h\).
Of course, we could pin down the position much better if we used \(N\) photons instead of a single one. From statistical theory, it is known that the remaining uncertainty \(\sim \lambda/\sqrt{N}\). But then \(N\) photons have bounced off the electron, so, since each is equally likely to have gone through any part of the object lens, the uncertainly in momentum of the electron as a consequence of these collisions goes up as \(\sqrt{N}\). (The same as the average imbalance between heads and tails in a sequence of \(N\) coin flips.)
Noting that the uncertainty in the momentum of the electron arises because we don’t know where the bounced-off photon passes through the object lens, it is tempting to think we could just use a smaller object lens, that would reduce \(\Delta p_x\). Although this is correct, recall from above that we stated the size of the diffraction pattern was \(\sim \lambda f/d\), where \(d\) is the diameter of the object lens and \(f\) its focal length. It is easy to see that the diffraction pattern, and consequently \(\Delta x\), gets bigger by just the amount that \(\Delta p_x\) gets smaller!
Watching Electrons in the Double Slit Experiment
Suppose now that in the double slit experiment, we set out to detect which slit each electron goes through by shining a light just behind the screen and watching for reflected light from the electron immediately after it had passed through a slit. Following the discussion in Feynman’s Lectures in Physics, Volume III, we shall now establish that if we can detect the electrons, we ruin the diffraction pattern!
Taking the distance between the two slits to be \(d\), the dark lines in the diffraction pattern are at angles \[ (n+\frac{1}{2})\lambda_{elec}=d\sin\theta \tag{1.4.5} \]
If the light used to see which slit the electron goes through generates an uncertainty in the electron’s y momentum \(\Delta p_y\)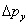 , in order not to destroy the diffraction pattern we must have \[ \Delta p_y/p <\lambda_{elec}/d \tag{1.4.6} \]
, in order not to destroy the diffraction pattern we must have \[ \Delta p_y/p <\lambda_{elec}/d \tag{1.4.6} \]
(the angular uncertainty in the electron’s direction must not be enough to spread it from the diffraction pattern maxima into the minima). Here \(p\) is the electron’s full momentum, \(p=h/\lambda_{elec}\). Now, the uncertainty in the electron’s y momentum, looking for it with a microscope, is \(\Delta p_y \sim h/\lambda_{light}\).
Substituting these values in the inequality above we find the condition for the diffraction pattern to survive is \[ \lambda_{light}>d \tag{1.4.7} \]
the wavelength of the light used to detect which slit the electron went through must be greater than the distance between the slits. Unfortunately, the light scattered from the electron then gives one point in a diffraction pattern of size the wavelength of the light used, so even if we see the flash this does not pin down the electron sufficiently to say which slit it went through. Heisenberg wins again.
How the Uncertainty Principle Determines the Size of Everything
It is interesting to see how the actual physical size of the hydrogen atom is determined by the wave nature of the electron, in effect, by the Uncertainty Principle. In the ground state of the hydrogen atom, the electron minimizes its total energy. For a classical atom, the energy would be minus infinity, assuming the nucleus is a point (and very large in any case) because the electron would sit right on top of the nucleus. However, this cannot happen in quantum mechanics. Such a very localized electron would have a very large uncertainty in momentum—in other words, the kinetic energy would be large. This is most clearly seen by imagining that the electron is going in a circular orbit of radius \(r\) with angular momentum \(h/2p\). Then one wavelength of the electron’s de Broglie wave just fits around the circle, \(\lambda_{elec}=2\pi r\). Clearly, as we shrink the circle’s radius \(r\), \(\lambda_{elec}\) goes down proportionately, and the electrons momentum
\[ p=h/\lambda_{elec}=h/2\pi r \tag{1.4.8} \]
increases. Adding the electron’s electrostatic potential energy we find the total energy for a circular orbit of radius \(r\) is:
\[ E(r)=K.E.+P.E.=\frac{p^2}{2m}-\frac{e^2}{4\pi \varepsilon_0r}=\frac{h^2}{8m\pi^2r^2}-\frac{e^2}{4\pi\varepsilon_0r} \tag{1.4.9} \]
Notice that for very large \(r\), the potential energy dominates, the kinetic energy is negligible, and shrinking the atom lowers the total energy. However, for small enough \(r\), the (always positive) kinetic energy term wins, and the total energy grows as the atom shrinks. Evidently, then, there must be a value of \(r\) for which the total energy is a minimum. Visualizing a graph of the total energy given by the equation above as a function of \(r\), at the minimum point the slope of \(E(r)\) is zero, \(dE(r)/dr=0\).
That is,
\[ -\frac{h^2}{4m\pi^2r}+\frac{e^2}{4\pi \varepsilon_0}=0 \tag{1.4.10} \]
giving
\[ r_{min}=\frac{\varepsilon_0h^2}{\pi me^2} \tag{1.4.11} \]
The total energy for this radius is the exact right answer, which is reassuring (but we don’t deserve it, because we have used a naïve picture, as will become clear later.)
The point of this exercise is to see that in quantum mechanics, unlike classical mechanics, a particle cannot position itself at the exact minimum of potential energy, because that would require a very narrow wave packet and thus be expensive in kinetic energy. The ground state of a quantum particle in an attractive potential is a trade off between potential energy minimization and kinetic energy minimization. Thus the physical sizes of atoms, molecules and ultimately ourselves are determined by Planck’s constant.
Contributor
- Michael Fowler (Beams Professor, Department of Physics, University of Virginia)


