4.6: The Hydrogen Atom
( \newcommand{\kernel}{\mathrm{null}\,}\)
Factoring Out the Center of Mass Motion
The hydrogen atom consists of two particles, the proton and the electron, interacting via the Coulomb potential V(→r1−→r2)=e2/r, where as usual r=|→r1−→r2|. Writing the masses of the two particles as m1, m2 Schrödinger’s equation for the atom is: (−ℏ22m1→∇12−ℏ22m2→∇22−e2r)ψ(→r1,→r2)=Eψ(→r1,→r2).
But →r1, →r2 are not the most natural position variables for describing this system: since the potential depends only on the relative position, a better choice is →r, →R defined by: →r=→r1−→r2,→R=m1→r1+m2→r2m1+m2
so →R is the center of mass of the system. It is convenient at the same time to denote the total mass by M=m1+m2, and the reduced mass by m=m1m2m1+m2.
Transforming in straightforward fashion to the variables →r, →R Schrödinger’s equation becomes (−ℏ22M→∇R2−ℏ22m→∇r2−e2r)ψ(→R, →r)=Eψ(→R, →r).
Writing the wave function ψ(→R, →r)=Ψ(→R)ψ(→r)
we can split the equation into two: (−ℏ22M→∇R2)Ψ(→R)=ERΨ(→R)(−ℏ22m→∇r2+V(→r))ψ(→r)=Erψ(→r)
and the total system energy is E=ER+Er. Note that the motion of the center of mass is (of course) just that of a free particle, having a trivial plane wave solution. From now on, we shall only be concerned with the relative motion of the particles. Since the proton is far heavier than the electron, we will almost always ignore the difference between the electron mass and the reduced mass, but it should be noted that the difference is easily detectable spectroscopically: for example, the lines shift if the proton is replaced by a deuteron (heavy hydrogen).
We’re ready to write Schrödinger’s equation for the hydrogen atom, dropping the r suffixes in the second equation above, and writing out →∇2 explicitly in spherical coordinates:
−ℏ22m(1r2∂∂r(r2∂ψ∂r)+1r2sinθ∂∂θ(sinθ∂ψ∂θ)+1r2sin2θ∂2ψ∂φ2)−e2rψ=Eψ.
Factoring Out the Angular Dependence: the Radial Equation - R(r)
Since the potential is spherically symmetric, the Hamiltonian H commutes with the angular momentum operators L2, Lz so we can construct a common set of eigenkets of the three operators H, L2, Lz. The angular dependence of these eigenkets is therefore that of the Yml’s, so the solutions must be of the form
ψElm(r,θ,ϕ)=RElm(r)Yml(θ,ϕ).
Now, notice that in the Schrödinger equation above, the angular part of →∇2 is exactly the differential operator L2/2mr2, so operating on ψElm(r,θ,ϕ)=RElm(r)Yml(θ,ϕ) it will give ℏ2l(l+1)/2mr2. The spherical harmonic Yml can then be cancelled from the two sides of the equation leaving:
−ℏ22m(1r2ddr(r2ddr)−l(l+1)r2)REl(r)−e2rREl(r)=EREl(r)
it now being apparent that R(r) cannot depend on m.
The radial derivatives simplify if one factors out 1/r from the function R, writing
REl(r)=u(r)r
and temporarily suppressing the E and l to reduce clutter.
The equation becomes:
−ℏ22m(d2dr2−l(l+1)r2)u(r)−e2ru(r)=Eu(r).
Rearranging,
−ℏ22md2u(r)dr2+(ℏ22ml(l+1)r2−e2r)u(r)=Eu(r).
Note that this is the same as the Schrödinger equation for a particle in one dimension, restricted to r>0, in a potential (for l≠0 ) going to positive infinity at the origin, then negative and going to zero at large distances, so it always has a minimum for some positive r.
We are interested in bound states of the proton-electron system, so E will be a negative quantity. At large separations, the wave equation simplifies to
−ℏ22md2u(r)dr2≅E u(r)(forlarger)
having approximate solutions eκr, e−κr where κ=√−2mE/ℏ2. The bound states we are looking for, of course, have exponentially decreasing wave functions at large distances.
Going to a Dimensionless Variable
To further simplify the equation, we introduce the dimensionless variable ρ=κr,κ=√−2mE/ℏ2
giving d2u(ρ)dρ2=(1−2νρ+l(l+1)ρ2)u(ρ)
where (for reasons which will become apparent shortly) we have introduced ν defined by 2ν=e2κ/E.
Notice that in transforming from r to the dimensionless variable ρ the scaling factor κ depends on energy, so will be different for different energy bound states!
Consider now the behavior of the wave function near the origin. The dominant term for sufficiently small ρ is the centrifugal one, so d2u(ρ)dρ2≅l(l+1)ρ2u(ρ)
for which the solutions are u(ρ)∼ρ−l, u(ρ)∼ρl+1. Since the wave function cannot be singular, we choose the second.
We have established that the wave function decays as e−κr=e−ρ at large distances, and goes as ρl+1 close to the origin. Factoring out these two asymptotic behaviors, define w(ρ) by u(ρ)=e−ρρl+1w(ρ).
It is straightforward (if tedious) to establish that w(ρ) satisfies the differential equation: ρd2w(ρ)dρ2+2(l+1−ρ)dw(ρ)dρ+2(ν−(l+1))w(ρ)=0.
Putting in a trial series solution w(ρ)=∑∞k=0wkρk gives a recurrence relation between successive coefficients: wk+1wk=2(k+l+1−ν)(k+1)(k+2(l+1)).
For large values of k, wk+1/wk→2/k, so wk∼2k/k! and therefore w(ρ)∼e2ρ. This means we have found the diverging radial wavefunction u(ρ)∼eρ, which is in fact the correct behavior for general values of the energy.
To find the bound states, we must choose energies such that the series is not an infinite one. As long as the series stops somewhere, the exponential decrease will eventually take over, and yield a finite (bound state) wave function. Just as for the simple harmonic oscillator, this can only happen if for some k, wk+1=0. Inspecting the ratio wk+1/wk, evidently the condition for a bound state is that ν=n,aninteger
in which case the series for w(ρ) terminates at k=n−l−1. From now on, since we know that for the functions we’re interested in ν is an integer, we replace ν by n.
To find the energies of these bound states, recall 2n=2ν=e2κ/E and κ=√−2mE/ℏ2, so 4n2=e4κ2nE2n=−e4E2n2mEnℏ2,
so En=−me42ℏ21n2=−13.6n2ev=−1n2Rydberg.
(This defines the Rydberg, a popular unit of energy in atomic physics.)
Remarkably, this is the very same series of bound state energies found by Bohr from his model! Of course, this had better be the case, since the series of energies Bohr found correctly accounted for the spectral lines emitted by hot hydrogen atoms. Notice, though, that there are some important differences with the Bohr model: the energy here is determined entirely by n, called the principal quantum number, but, in contrast to Bohr’s model, n is not the angular momentum. The true ground state of the hydrogen atom, n=1, has zero angular momentum: since n=k+l+1, n=1 means both l=0 and k=0. The ground state wave function is therefore spherically symmetric, and the function w(ρ)=w0 is just a constant. Hence u(ρ)=ρe−ρw0 and the actual radial wave function is this divided by r, and of course suitably normalized.
To write the wave function in terms of r, we need to find κ. Putting together ρ=κnr, κn=√−2mEn/ℏ2 and En=−me42ℏ21n2, κn=√2mme42ℏ21n2/ℏ=me2ℏ2n=1a0n,
where the length a0=ℏ2me2=0.529×10−10m.
is called the Bohr radius: it is in fact the radius of the lowest orbit in Bohr’s model.
Exercise: check this last statement.
It is worth noting at this point that the energy levels can be written in terms of the Bohr radius a0: En=−e22a01n2.
(This is actually obvious: remember that the energies En are identical to those in the Bohr model, in which the radius of the nth orbit is n2a0, so the electrostatic potential energy is −e2/na0, etc.)
Moving on to the excited states: for n=2, we have a choice: either the radial function w(ρ) can have one term, as before, but now the angular momentum l=1 (since n=k+l+1 ); or w(ρ) can have two terms (so k=1 ), and l=0. Both options give the same energy, -0.25 Ry, since n is the same, and the energy only depends on n. In fact, there are four states at this energy, since l=1 has states with m=1, m=0 and m=−1, and l=0 has the one state m=0. (For the moment, we are not counting the extra factor of 2 from the two possible spin orientations of the electron.)
For n=3, there are 9 states altogether: gives one, l=1 gives 3 and l=2 gives 5 different m values. In fact, for principal quantum number n there are n2 degenerate states. ( n2 being the sum of the first n odd integers.)
The states can be mapped out, energy vertically, angular momentum horizontally:
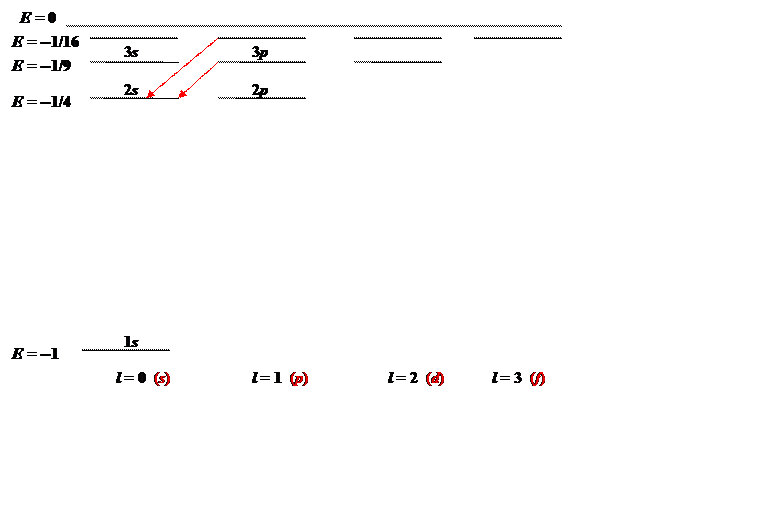
The energy E=−1/n2, the levels are labeled nl, n being the principal quantum number and the traditional notation for angular momentum l is given at the bottom of the diagram. The two red vertical arrows are the first two transitions in the spectroscopic Balmer series, four lines of which gave Bohr the clue that led to his model. The corresponding series of transitions to the 1s ground state are in the ultraviolet, they are called the Lyman series.
Wave Functions for some Low-n States
From now on, we label the wave functions with the quantum numbers, ψnlm(r,θ,ϕ), so the ground state is the spherically symmetric ψ100(r).
For this state R(r)=u(r)/r, where u(ρ)=e−ρρl+1w(ρ)=e−ρρw0, with w0 a constant, and ρ=κ1r=r/a0.
So, as a function of r, ψ100(r)=Ne−r/a0 with N an easily evaluated normalization constant:
ψ100(r)=(1πa30)1/2e−r/a0.
For n=2, l=1 the function w(ρ) is still a single term, a constant, but now u(ρ)=e−ρρl+1w(ρ)=e−ρρ2w0, and, for n=2, ρ=κr=r/2a0, remembering the energy-dependence of κ.
Therefore ψ210(r,θ,ϕ)=N(ra0)e−r/2a0cosθ. Again, evaluating the normalization constant is routine, yielding ψ210(r,θ,ϕ)=(132πa30)1/2(ra0)e−r/2a0cosθ.
The wave functions for the other m -values, ψ21±1(r,θ,ϕ), have the cosθ in ψ210 replaced by ∓(1/√2)sinθe±iϕ respectively (from the earlier discussion of the Yml ’s).
The other n=2 state has l=0, so from n=k+l+1, we have k=1 and the series for w has two terms, k=0 and k=1, the ratio being
wk+1wk=2(k+l+1−n)(k+1)(k+2(l+1))=−1
for the relevant values: k=0, l=0, n=2. So w1=−w0, w(ρ)=w0(1−ρ). For n=2, ρ=r/2a0, the normalized wave function is
ψ200(r)=(132πa30)1/2(2−ra0)e−r/2a0.
Note that the zero angular momentum wave functions are nonzero and have nonzero slope at the origin. This means that the full three dimensional wave functions have a slope discontinuity there! But this is fine—the potential is infinite at the origin. (Actually, the proton is not a point charge, so really the kink will be smoothed out over a volume of the size of the proton—a very tiny effect.)
General Solution of the Radial Equation
In practice, the first few radial functions w(ρ) can be constructed fairly easily using the method presented above, but it should be noted that the differential equation for w(ρ) ρd2w(ρ)dρ2+2(l+1−ρ)dw(ρ)dρ+2(n−(l+1))w(ρ)=0
is in fact Laplace’s equation, usually written (zd2dz2+(k−1−z)ddz+p)Lkp(z)=0
where k,p are integers, and Lkp(z) is a Laguerre polynomial (Messiah, page 482).
The two equations are the same if z=2ρ, and the solution to the radial equation is therefore wnl(ρ)=L2l+1n−l−1(2ρ).
Quoting Messiah, the Laguerre polynomials L0p(z), and the associated Laguerre polynomials Lkp(z) are given by:
L0p(z)=ezdpdzpe−zzpLkp(z)=(−1)kdkdzkL0p+k(z).
(These representations can be found neatly by solving Laplace’s equation using – surprise – a Laplace transform. See Merzbacher for details.) The polynomials satisfy the orthonormality relations (with the mathematicians’ normalization convention)
∫∞0e−zzkLkpLkqdz=[(p+k)!]3p!δpq.
But what do they look like? The function e−zzp is zero at the origin (apart from the trivial case p=0 ) and zero at infinity, always positive and having nonzero slope except at its maximum value, z=p. The p derivatives bring in p separated zeroes, easily checked by sketching the curves generated by successive differentiation. Therefore, L0p(z), a polynomial of degree p, has p real positive zeroes, and value at the origin L0p(0)=p!, since the only nonzero term at z=0 is that generated by all p differential operators acting on zp.
The associated Laguerre polynomial Lkp(z) is generated by differentiating L0p+k(z) k times. Now L0p+k(z) has p+k real positive zeroes, differentiating it gives a polynomial one degree lower, with zeroes which must be one in each interval between the zeroes of L0p+k(z). This argument remains valid for successive differentiations, so Lkp(z) must have p real separate zeroes.
Putting all this together, and translating back from ρ to r, the radial solutions are:
Rnl(r)=Ne−r/na0(rna0)lL2l+1n−l−1(2rna0)
with N the normalization constant. Griffiths (page 141) gives more details, including the normalization constants worked out. We used those to plot the n=3 states—plotting here the functions u(r)=rR(r), since the normalization is 4π∫∞0|u(r)|2dr=1,u(r) gives a better idea of at what distance from the proton the electron is most likely to be found.
Here are the three n=3 radial wave functions:
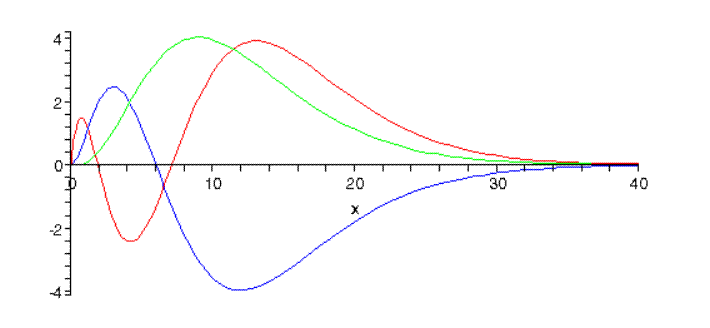
The number of nodes, the radial quantum number, is 3−l−1. (Note: The relative normalizations are correct here, but not the overall normalization.)
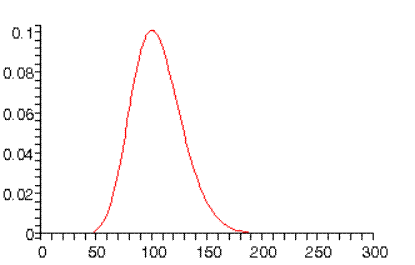
For higher n values, the wave functions become reminiscent of classical mechanics. for example, for n=10, the highest angular momentum state probability distribution peaks at r=100a0, the Bohr orbit radius:
whereas for n=10, l=0, we find:
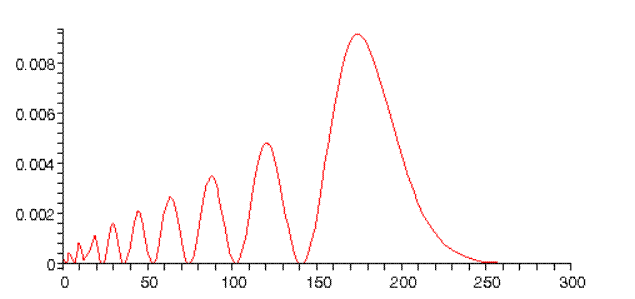
Notice this peaks just below twice the Bohr radius. This can be understood from classical mechanics: for an inverse square force law, elliptical orbits with the same semimajor axis have the same energy. The l=n−1 orbit is a circle, the l=0 orbit is a long thin ellipse (one end close to the proton), so it extends almost twice as far from the origin as the circle. Furthermore, the orbiting electron will spend longer at the far distance, since it will be moving very slowly. (Note: the normalizations in the above graphs are only approximate.)


