16.2: The Coulomb force
( \newcommand{\kernel}{\mathrm{null}\,}\)
Coulomb was the first to provide a detailed quantitative description of the force between charged objects. Nowadays, we use the (derived) SI unit of “Coulomb” (C) to represent charge. The “charge” of an object corresponds to the net excess (or lack) of electrons on the object. An electron has a charge of −e=−1.6×10−19C. Thus, an object with a charge of −1C has an excess of about −1.6×1019 electrons on it, which is a very large charge. If an object has an excess of electrons, it is negatively charged and we indicate this with a negative sign on the charge of the object. An object with a (positive) charge of 1C thus has a deficit of −1.6×1019 electrons.
Through careful studies of the force between two charged spheres, Coulomb observed1 that:
- The force is attractive if the objects have opposite charges and repulsive if the objects have the same charge.
- The force is inversely proportional to the squared distance between spheres.
- The force is larger if the charges involved are larger.
This leads to Coulomb’s Law for the electric force (or simply “Coulomb’s Law”), →F12, exerted on a point charge Q1 by another point charge Q2:
→F12=kQ1Q2r2ˆr21
where ˆr21 is the unit vector from Q2 to Q1 and r is the distance between the two charges, as illustrated in Figure 16.2.1. k=8.99×109N⋅m2/C2 is simply a proportionality constant (“Coulomb’s constant”) to ensure that the quantity on the right will have units of Newtons when all other quantities are in S.I. units. In some instances, it is more convenient to use the “permittivity of free space”, ϵ0, rather than Coulomb’s constant, in which case Coulomb’s Law has the form:
→F12=14πϵ0Q1Q2r2ˆr21
where ϵ0=14πk=8.85×10−12C2⋅N−1⋅m−2 is a more fundamental constant, as we will see in later chapters.
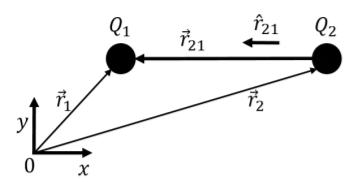
If the two charges have positions →r1 and →r2, respectively, then the vector ˆr21 is given by:
ˆr21=→r2−→r1||→r2−→r1||
Coulomb’s Law is mathematically identical to the gravitational force in Newton’s Universal Theory of Gravity. Rather than quantity of mass determining the strength of the gravitational force, it is the quantity of charge that determines the strength of the electric force. The only major difference is that gravity is always attractive, whereas the Coulomb force can be repulsive.
The Coulomb force is conservative.
- True.
- False.
- Answer
- A.
The product Q1Q2 in the numerator of Coulomb’s force is positive if the two charges have the same sign (both positive or both negative) and negative if the charges have opposite signs. Again, referring to Figure 16.2.1, if the two charges are positive, the force on Q1 will point in the same direction as ˆr21 (since all of the scalars are positive in Coulomb’s Law) and thus be repulsive. If, instead, the two charges have opposite signs, the product Q1Q2 will be negative and the force vector on Q1 will point in the opposite direction from ˆr21 and the force is attractive.
Calculate the magnitude of the electric force between the electron and the proton in a hydrogen atom and compare this to the gravitational force between them.
Solution
We model this by assuming that the electron and proton are point charges a distance of 1Å=1×10−10m apart (1 Angstrom is about the size of the hydrogen atom). The proton and electron have the same charge with magnitude e=1.6×10−19C, so the (attractive) electric force between them has a magnitude of:
Fe=kQ1Q2r2=(9×109N⋅m2/C2)(1.6×10−19C)(1.6×10−19C)(1×10−10m)2=2.3×10−8N
which is a small number, but acting on a very small mass. In comparison, the force of gravity between an electron (me=9.1×10−31kg) and a proton (mp=1.7×10−27kg) is given by:
Fg=Gm2mpr2=(6.7×10−11Nm2/kg2(9.1×10−31kg)(1.7×10−27kg)(1×10−10m)2=1.04×10−47N
Discussion
As we can see, the electric force between an electron and a proton is 39 orders of magnitude larger than the gravitational force! This shows that the gravitational force is extremely weak on the scale of particles and has essentially no effect in particle physics. Indeed, the best current theory of particle physics, and the most precisely tested theory in physics, the “Standard Model”, does not need to include gravity in order to provide a spectacularly precise description of particles. One of the big challenges in theoretical physics is nonetheless to develop a theory that integrates the gravitational force with the other forces.
Three charges, Q1=1nC, Q2=−2nC, and q=−1nC, are held fixed at the three corners of an equilateral triangle with sides of length a=1cm, with a coordinate system as shown in Figure 16.2.2. What is the electric force vector on charge q? (Note that 1nC=1×10−9C).
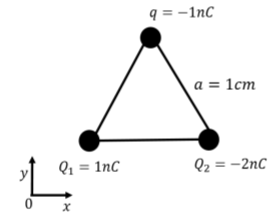
Solution
The net electric force on charge q will be the vector sum of the forces from charges Q1 and Q2. We thus need to determine the force vectors on q from each charge using Coulomb’s Law, and then add those two vectors to obtain the net force on q. The force vectors exerted on q from each charge are illustrated in Figure 16.2.3.
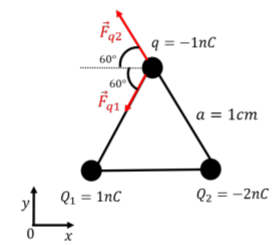
The force from charge Q1 has magnitude:
Fq1=|kQ1qa2|=(9×109N⋅M2/C2)(1×10−9C)(1×10−9C)(0.01m)2=9×10−5N
and components:
→Fq1=−Fq1cos(60∘)ˆx−Fq1sin(60∘)ˆy=−(4.5×10−5N)ˆx−(7.8×10−5N)ˆy
Similarly, the force on q from Q2 has magnitude:
Fq2=|kQ2qa2|=(9×109N⋅m2/C2)(2×10−9C)(1×10−9C)(0.01m)2=1.8×10−4N
and components:
→Fq2=−Fq2cos(60∘)ˆx+Fq2sin(60∘)ˆy=−(9.0×10−5N)ˆx+(1.6×10−4N)ˆy
Finally, we can add the two force vectors together to obtain the net force on q:
→Fnet=→Fq1+→Fq2=−(4.5×10−5N)ˆx−(7.8×10−5N)ˆy−(9.0×10−5N)ˆx+(1.6×10−4N)ˆy=−(13.5×10−5N)ˆx+(8.2×10−5N)ˆy
which has a magnitude of 15.8×10−5N.
Discussion
In this example, we determined the net force on a charge by making use of the superposition principle; namely, that we can treat the forces exerted on q by Q1 and Q2 independently, without needing to consider the fact that Q1 and Q2 exert forces on each other.
Footnotes
1. Others had initially observed the inverse square law for the electric force, but Coulomb was the first to formalize the theory.

