2.4: Electrostatic Force - Coulomb's Law
( \newcommand{\kernel}{\mathrm{null}\,}\)
Coulomb's Force
Experiments with electric charges have shown that if two objects each have electric charge, then they exert an electric force on each other. The magnitude of the force is linearly proportional to the net charge on each object and inversely proportional to the square of the distance between them. (Interestingly, the force does not depend on the mass of the objects.) The direction of the force vector is along the imaginary line joining the two objects and is dictated by the signs of the charges involved.
Let
- q1,q2= the net electric charge of the two objects;
- →r12= the vector displacement from q1 to q2.
The electric force →F on one of the charges is proportional to the magnitude of its own charge and the magnitude of the other charge, and is inversely proportional to the square of the distance between them:
F∝q1q2r212.
This proportionality becomes an equality with the introduction of a proportionality constant:
The electric force (or Coulomb force) experienced by charge q2 due to charge q1 is equal to
→F12=14πε0 q1 q2r212ˆr12=14πε0 q1 q2r312→r12
The unit vector ˆr12=→r12|→r12| has a magnitude of 1 and points along the axis as the charges from q1 to q2 (same direction as vector →r12).

If the charges have the same sign, the force is in the same direction as →r12 showing a repelling force. If the charges have different signs, the force is in the opposite direction of →r12 showing an attracting force. (Figure 2.4.1).

It is important to note that the electric force is not constant; it is a function of the separation distance between the two charges. If either the test charge or the source charge (or both) move, then →r changes, and therefore so does the force. An immediate consequence of this is that direct application of Newton’s laws with this force can be mathematically difficult, depending on the specific problem at hand. It can (usually) be done, but we almost always look for easier methods of calculating whatever physical quantity we are interested in. (Conservation of energy is the most common choice.)
Finally, the new constant ϵ0 in Coulomb’s law is called the permittivity of free space, or (better) the permittivity of vacuum. It has a very important physical meaning that we will discuss in a later chapter; for now, it is simply an empirical proportionality constant. Its numerical value (to three significant figures) turns out to be
ϵ0=8.85×10−12C2N⋅m2.
These units are required to give the force in Coulomb’s law the correct units of newtons. Note that in Coulomb’s law, the permittivity of vacuum is only part of the proportionality constant. For convenience, we often define a Coulomb’s constant:
ke=14πϵ0=8.99×109N⋅m2C2.
Examples
A hydrogen atom consists of a single proton and a single electron. The proton has a charge of +e and the electron has −e. In the “ground state” of the atom, the electron orbits the proton at most probable distance of 5.29×10−11m (Figure 2.4.2). Calculate the electric force on the electron due to the proton.
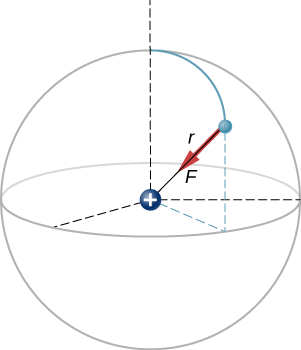
- Strategy
-
For the purposes of this example, we are treating the electron and proton as two point particles, each with an electric charge, and we are told the distance between them; we are asked to calculate the force on the electron. We thus use Coulomb’s law (Equation ???).
- Solution
-
Our two charges are,
q1=+e=+1.602×10−19Cq2=−e=−1.602×10−19C
and the distance between them
r=5.29×10−11m.
The magnitude of the force on the electron (Equation ???) is
F=14πϵ0|q1q2|r212=14π(8.85×10−12C2N⋅m2)(1.602×10−19C)2(5.29×10−11m)2=8.25×10−8
As for the direction, since the charges on the two particles are opposite, the force is attractive; the force on the electron points radially directly toward the proton, everywhere in the electron’s orbit. The force is thus expressed as
→F=−(8.25×10−8N)ˆr.
You might have noticed that Coulomb's force bears a striking resemblance to the Universal Gravitational Expression between two masses m1 and m2
→F12=−G m1 m2r212ˆr12=−G m1 m2r312→r12
Multiple Source Charges
The analysis that we have done for two particles can be extended to an arbitrary number of particles; we simply repeat the analysis, two charges at a time. Specifically, we ask the question: Given N charges (which we refer to as source charge), what is the net electric force that they exert on some other point charge (which we call the test charge)? Note that we use these terms because we can think of the test charge being used to test the strength of the force provided by the source charges.
Like all forces that we have seen up to now, the net electric force on our test charge is simply the vector sum of each individual electric force exerted on it by each of the individual test charges. Thus, we can calculate the net force on the test charge Q by calculating the force on it from each source charge, taken one at a time, and then adding all those forces together (as vectors). This ability to simply add up individual forces in this way is referred to as the principle of superposition, and is one of the more important features of the electric force. In mathematical form, this becomes
→F(r)=14πϵ0QN∑i=1qir2iˆr2i.
In this expression, Q represents the charge of the particle that is experiencing the electric force →F, and is located at →r from the origin; the q′is are the N source charges, and the vectors →ri=riˆri are the displacements from the position of the ith charge to the position of Q. Each of the N unit vectors points directly from its associated source charge toward the test charge. All of this is depicted in Figure 2.4.2. Please note that there is no physical difference between Q and qi; the difference in labels is merely to allow clear discussion, with Q being the charge we are determining the force on.
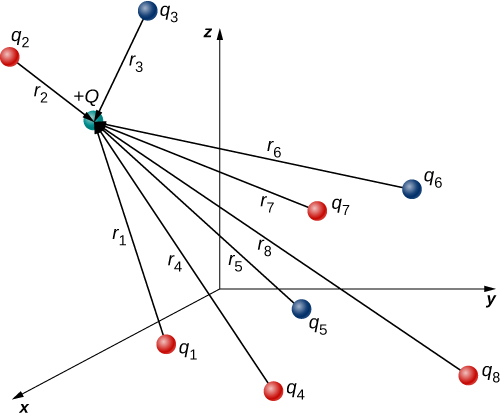
(Note that the force vector →Fi does not necessarily point in the same direction as the unit vector ˆri; it may point in the opposite direction, −ˆri. The signs of the source charge and test charge determine the direction of the force on the test charge.)
There is a complication, however. Just as the source charges each exert a force on the test charge, so too (by Newton’s third law) does the test charge exert an equal and opposite force on each of the source charges. As a consequence, each source charge would change position. However, by Equation ???, the force on the test charge is a function of position; thus, as the positions of the source charges change, the net force on the test charge necessarily changes, which changes the force, which again changes the positions. Thus, the entire mathematical analysis quickly becomes intractable. Later, we will learn techniques for handling this situation, but for now, we make the simplifying assumption that the source charges are fixed in place somehow, so that their positions are constant in time. (The test charge is allowed to move.) With this restriction in place, the analysis of charges is known as electrostatics, where “statics” refers to the constant (that is, static) positions of the source charges and the force is referred to as an electrostatic force.
Examples
Three different, small charged objects are placed as shown in Figure 2.4.2. The charges q1 and q3 are fixed in place; q2 is free to move. Given q1=2e,q2=−3e, and q3=−5e, and that d=2.0×10−7m, what is the net force on the middle charge q2?

- Strategy
-
We use Coulomb’s law again. The way the question is phrased indicates that q2 is our test charge, so that q1 and q3 are source charges. The principle of superposition says that the force on q2 from each of the other charges is unaffected by the presence of the other charge. Therefore, we write down the force on q2 from each and add them together as vectors.
- Solution
-
We have two source charges q1 and q3 a test charge q2, distances r21 and r23 and we are asked to find a force. This calls for Coulomb’s law and superposition of forces. There are two forces:
→F2=→F12+→F32=14πϵ0[(−q2q1r212ˆj)+(−q2q3r232ˆi)].
We cannot add these forces directly because they don’t point in the same direction: →F12 points only in the +y-direction, while →F32 points only in the -x-direction. The net force is obtained from applying the Pythagorean theorem to its x- and y-components:
F2=√F22x+F22y
Thus:
→F2=14πϵ0[(−q2q3r232ˆi)+(−q2q1r212ˆj)].
→F2=(8.99×109N⋅m2C2)[(−(−4.806×10−19C)(−8.01×10−19C)(4.00×10−7m)2ˆi)+(−(−4.806×10−19C)(3.204×10−19C)(2.00×10−7m)2ˆj)].=−(2.16×10−14N)ˆi+(3.46×10−14N)ˆj.
We find thatF2=√F22x+F22y=4.08×10−14N
at an angle of
ϕ=tan−1(F2yF2x)=tan−1(3.46×10−14N−2.16×10−14N)=−58o,
that is, 58o above the −x-axis, as shown in the diagram.
Shown below are 4 identical positive charges located at the corners of a square. The magnitude of the force on charge 1 by charge 4 is 4.0N. Find the magnitude of the total force on charge 3 exerted by charges 1, 2, and 4.
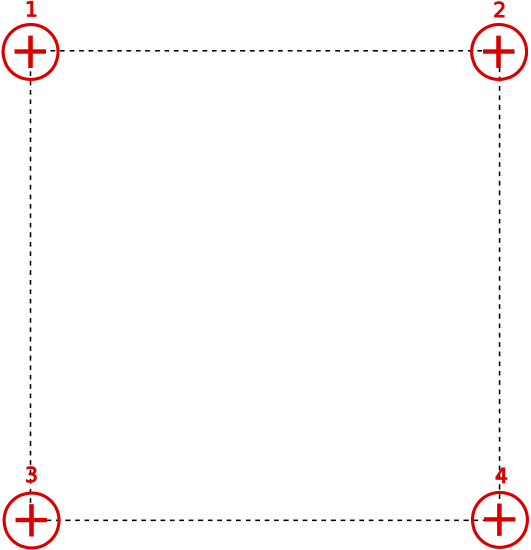
- Solution
-
Let the side of the square be distance a. The relevant distances and forces on charge 3 are shown below.
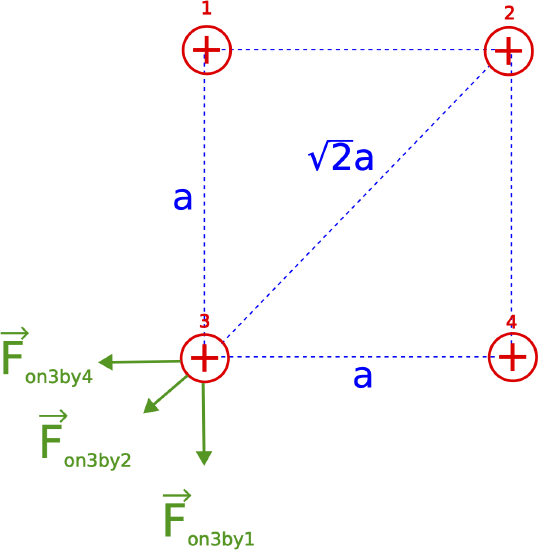
Since the magnitude of the force on charge 1 by charge 4 is 4 N, the same as the magnitude on charge 3 by charge 2 is also 4 N, since the distance between 1 and 4 is the same as between 2 and 3, and the charges are identical, all positive with the same charge q. Thus, the magnitude of the force on 3 by 2 is given by:
|→Fon 3 by 2|=kq2a2=4N
The magnitude of the force on 2 by both 1 and 4 are the same since the distance is the same:
|→Fon 3 by 1|=|→Fon 3 by 4|=kqa2
Comparing the two equations above we see that the force by 1 and 4 is double the force by 2. Therefore:
|→Fon 3 by 1|=|→Fon 3 by 4|=8N
The direction of the force by 1 is down since the forces are repulsive. In vector form this is written as:
→Fon 3 by 1=(0,−8)N
The direction of the force by 4 is to the left:
→Fon 3 by 4=(−8,0)N
These two vectors combined are:
→Fon 3 by 1+→Fon 3 by 4=(−8,−8)N
One way to continue is to break down →Fon 3 by 2 into components, then add to the sum of the two other forces above, and then find the magnitude. But there is a convenient shortcut when we recognize that the combined vector above will point in the same direction as →Fon 3 by 2, thus you can just add their magnitudes (it's now a 1D vector addition) to get the total magnitude of the force:
|→Fnet|=√82+82+4=15.3N
Force Due to a Continuous Charge Distribution
The charge distributions we have seen so far have been discrete: made up of individual point particles. This is in contrast with a continuous charge distribution, which has at least one nonzero dimension. If a charge distribution is continuous rather than discrete, we can generalize the definition of the electric field. We simply divide the charge into infinitesimal pieces and treat each piece as a point charge.
Note that because charge is quantized, there is no such thing as a “truly” continuous charge distribution. However, in most practical cases, the total charge creating the field involves such a huge number of discrete charges that we can safely ignore the discrete nature of the charge and consider it to be continuous. This is exactly the kind of approximation we make when we deal with a bucket of water as a continuous fluid, rather than a collection of H2O molecules.
Our first step is to define a charge density for a charge distribution along a line, across a surface, or within a volume, as shown in Figure 2.4.1.
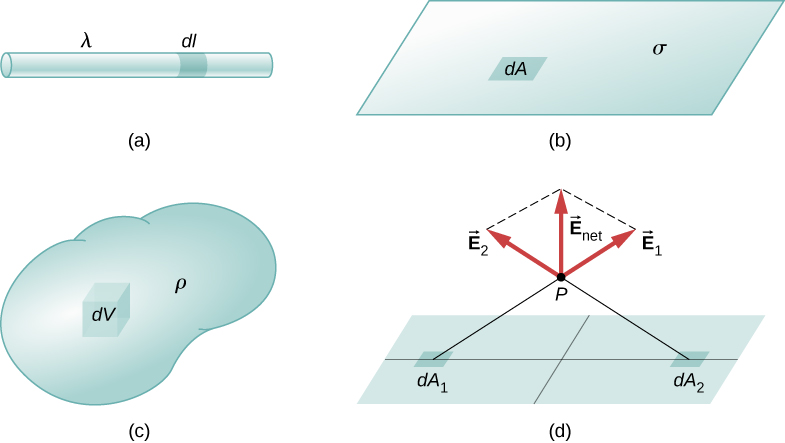
Definitions of charge density:
- linear charge density: λ≡ charge per unit length (Figure 2.4.5a); units are coulombs per meter (C/m)
- surface charge density: σ≡ charge per unit area (Figure 2.4.5b); units are coulombs per square meter (C/m2)
- volume charge density: ρ≡ charge per unit volume (Figure 2.4.5c); units are coulombs per square meter (C/m3)
For a line charge, a surface charge, and a volume charge, the summation in the definition of an Electric field discussed previously becomes an integral and qi is replaced by dq=λdl, σdA, or ρdV, respectively:
A point charge q is placed a distance z above the center of a ring that has a uniform charge density λ, with units of coulomb per unit meter of arc, resulting in a total charge of Q. Find the electric force acting on the charge q.
- Strategy
-
We use the same procedure as for multiple charges. The difference here is that the multiple charges is a charge that is distributed on a circle. We divide the circle into infinitesimal elements shaped as arcs on the circle each having a charge dq and use polar coordinates shown in Figure 2.4.4.

Figure 2.4.4: The system and variable for calculating the electric force due to a ring of charge. - Solution
-
The electric force on q due to an arc line charge dq is given by Coulomb's Law:
d→Fq=14πϵ0dq qr2ˆr.
The charge dq in the arc line charge dl spanning the angle dθ is given by:
dq=λ dl=λ (R dθ).
The force is then given by:
d→Fq=14πϵ0λ R dθ qr2ˆr.
The force due to the whole ring can be obtained through integration:
→Fq=14πϵ0∫lineλ R dθ qr2ˆr.
A general element of the arc between θ and θ+dθ is of length Rdθ and therefore contains a charge equal to λRdθ. The element is at a distance of r=√z2+R2 from P, the angle is cosϕ=z√z2+R2 and therefore the electric field is
→E(P)=14πϵ0∫lineλdlr2ˆr=14πϵ0∫2π0λRdθz2+R2z√z2+R2ˆz=14πϵ0λRz(z2+R2)3/2ˆz∫2π0dθ=14πϵ02πλRz(z2+R2)3/2ˆz=14πϵ0qtotz(z2+R2)3/2ˆz.
Significance
As usual, symmetry simplified this problem, in this particular case resulting in a trivial integral. Also, when we take the limit of z≫R, we find that
→E≈14πϵ0qtotz2ˆz,
as we expect.
Find the electric force on a charge q placed at the origin (point P), a distance a to the left of a straight line segment of length L=b−a that carries a uniform positive line charge density λ and overall charge Q.

- Strategy
-
Since this is a continuous charge distribution, we conceptually break the wire segment into differential pieces of length dl, each of which carries a differential amount of charge
dq=λdl.
Then, we conclude that because of symmetry, the force due to each segment is along the x-axis and directed either towards (−ˆi) or (+ˆi) depending on the signs of the charges q and Q. We then integrate the differential force expression over the length of the wire, from x=a to x=b to obtain the complete electric force expression.

Figure 2.4.4: A uniformly charged segment of wire. The electric Force on charge q can be found by applying the superposition principle to charge elements and integrating. - Solution
-
The electric force due to a line charge segment dl on the charge q is given by the general Coulomb expression:
d→Fq=14πϵ0(λ dl) qr2ˆi.
The electric force due to the whole line charge segment on the charge q can be found through integration over the whole line segment:
→Fq=14πϵ0∫line(λ dl) qr2ˆi.
The symmetry of the situation implies that there are only horizontal (x)-components of the Force. The drawing implies that the charges are identical in sign resulting in a repulsive force. However, the calculation will maintain the vector notation to ensure that the final expression will work for whatever charge sign configuration.
So we have
→Fq=−14πϵ0∫(λdl) qr2ˆi=−14πϵ0∫ba(λdx) qx2ˆi
where our differential line element dl is dx, in this example, since we are integrating along a line of charge that lies on the x-axis. (The limits of integration are a to b.
→Fq=14πϵ0∫ba(λdx) qx2ˆi=λ q4πϵ0[1x]baˆi=λ q4πϵ0[−1b−−1a]ˆi=λ q4πϵ0[1a−1b]ˆi.
The result is then:
→Fq=λ q4πϵ0(1a−1b)ˆi.


