1.4: Units and Standards
- Page ID
- 68422
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how SI base units are defined.
- Describe how derived units are created from base units.
- Express quantities given in SI units using metric prefixes.
Order of Magnitude
The order of magnitude of a number is the power of 10 that most closely approximates it. Thus, the order of magnitude refers to the scale (or size) of a value. Each power of 10 represents a different order of magnitude. For example, 101, 102, 103, and so forth, are all different orders of magnitude, as are 100 = 1, 10−1, 10−2, and 10−3. To find the order of magnitude of a number, take the base-10 logarithm of the number and round it to the nearest integer, then the order of magnitude of the number is simply the resulting power of 10. For example, the order of magnitude of 800 is 103 because log10 800 ≈ 2.903, which rounds to 3. Similarly, the order of magnitude of 450 is 103 because log10 450 ≈ 2.653, which rounds to 3 as well. Thus, we say the numbers 800 and 450 are of the same order of magnitude: 103. However, the order of magnitude of 250 is 102 because log10 250 ≈ 2.397, which rounds to 2.
An equivalent but quicker way to find the order of magnitude of a number is first to write it in scientific notation and then check to see whether the first factor is greater than or less than \(\sqrt{10}\) = 100.5 ≈ 3. The idea is that \(\sqrt{10}\) = 100.5 is halfway between 1 = 100 and 10 = 101 on a log base-10 scale. Thus, if the first factor is less than \(\sqrt{10}\), then we round it down to 1 and the order of magnitude is simply whatever power of 10 is required to write the number in scientific notation. On the other hand, if the first factor is greater than \(\sqrt{10}\), then we round it up to 10 and the order of magnitude is one power of 10 higher than the power needed to write the number in scientific notation. For example, the number 800 can be written in scientific notation as 8 x 102. Because 8 is bigger than \(\sqrt{10}\) ≈ 3, we say the order of magnitude of 800 is 102 + 1 = 103. The number 450 can be written as 4.5 x 102, so its order of magnitude is also 103 because 4.5 is greater than 3. However, 250 written in scientific notation is 2.5 x 102 and 2.5 is less than 3, so its order of magnitude is 102.
The order of magnitude of a number is designed to be a ballpark estimate for the scale (or size) of its value. It is simply a way of rounding numbers consistently to the nearest power of 10. This makes doing rough mental math with very big and very small numbers easier. For example, the diameter of a hydrogen atom is on the order of 10−10 m, whereas the diameter of the Sun is on the order of 109 m, so it would take roughly 109/10−10 = 1019 hydrogen atoms to stretch across the diameter of the Sun. This is much easier to do in your head than using the more precise values of 1.06 x 10−10 m for a hydrogen atom diameter and 1.39 x 109 m for the Sun’s diameter, to find that it would take 1.31 x 1019 hydrogen atoms to stretch across the Sun’s diameter. In addition to being easier, the rough estimate is also nearly as informative as the precise calculation.
Known Ranges of Length, Mass, and Time
The vastness of the universe and the breadth over which physics applies are illustrated by the wide range of examples of known lengths, masses, and times (given as orders of magnitude) in Figure \(\PageIndex{3}\). Examining this table will give you a feeling for the range of possible topics in physics and numerical values. A good way to appreciate the vastness of the ranges of values in Figure \(\PageIndex{3}\) is to try to answer some simple comparative questions, such as the following:
- How many hydrogen atoms does it take to stretch across the diameter of the Sun?
- How many protons are there in a bacterium?
- How many floating-point operations can a supercomputer do in 1 day?
- Answer a
-
109 m/10–10 m = 1019 hydrogen atoms
- Answer b
-
10–15 kg/10–27 kg = 1012 protons
- Answer c
-
105 s/10–17 s = 1022 floating-point operations
In studying Figure \(\PageIndex{3}\), take some time to come up with similar questions that interest you and then try answering them. Doing this can breathe some life into almost any table of numbers.
The range of objects and phenomena studied in physics is immense. From the incredibly short lifetime of a nucleus to the age of Earth, from the tiny sizes of subnuclear particles to the vast distance to the edges of the known universe, from the force exerted by a jumping flea to the force between Earth and the Sun, there are enough factors of 10 to challenge the imagination of even the most experienced scientist. Giving numerical values for physical quantities and equations for physical principles allows us to understand nature much more deeply than qualitative descriptions alone. To comprehend these vast ranges, we must also have accepted units in which to express them. We shall find that even in the potentially mundane discussion of meters, kilograms, and seconds, a profound simplicity of nature appears: all physical quantities can be expressed as combinations of only seven base physical quantities.
Units and Standards
We define a physical quantity either by specifying how it is measured or by stating how it is calculated from other measurements. For example, we might define distance and time by specifying methods for measuring them, such as using a meter stick and a stopwatch. Then, we could define average speed by stating that it is calculated as the total distance traveled divided by time of travel.
Measurements of physical quantities are expressed in terms of units, which are standardized values. For example, the length of a race, which is a physical quantity, can be expressed in units of meters (for sprinters) or kilometers (for distance runners). Without standardized units, it would be extremely difficult for scientists to express and compare measured values in a meaningful way (Figure \(\PageIndex{1}\)).
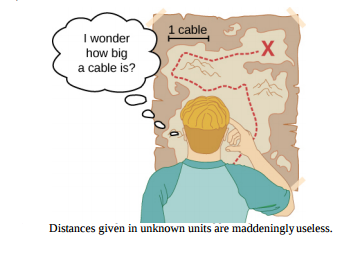
Two major systems of units are used in the world: SI units (for the French Système International d’Unités), also known as the metric system, and English units (also known as the customary or imperial system). English units were historically used in nations once ruled by the British Empire and are still widely used in the United States. English units may also be referred to as the foot–pound–second (fps) system, as opposed to the centimeter–gram–second (cgs) system. You may also encounter the term SAE units, named after the Society of Automotive Engineers. Products such as fasteners and automotive tools (for example, wrenches) that are measured in inches rather than metric units are referred to as SAE fasteners or SAE wrenches.
Virtually every other country in the world (except the United States) now uses SI units as the standard. The metric system is also the standard system agreed on by scientists and mathematicians.
SI Units: Base and Derived Units
In any system of units, the units for some physical quantities must be defined through a measurement process. These are called the base quantities for that system and their units are the system’s base units. All other physical quantities can then be expressed as algebraic combinations of the base quantities. Each of these physical quantities is then known as a derived quantity and each unit is called a derived unit. The choice of base quantities is somewhat arbitrary, as long as they are independent of each other and all other quantities can be derived from them. Typically, the goal is to choose physical quantities that can be measured accurately to a high precision as the base quantities. The reason for this is simple. Since the derived units can be expressed as algebraic combinations of the base units, they can only be as accurate and precise as the base units from which they are derived.
Based on such considerations, the International Standards Organization recommends using seven base quantities, which form the International System of Quantities (ISQ). These are the base quantities used to define the SI base units. Table \(\PageIndex{1}\) lists these seven ISQ base quantities and the corresponding SI base units.
| ISQ Base Quantity | SI Base Unit |
|---|---|
| Length | meter (m) |
| Mass | kilogram (kg) |
| Time | second (s) |
| Electrical Current | ampere (A) |
| Thermodynamic Temperature | kelvin (K) |
| Amount of Substance | mole (mol) |
| Luminous Intensity | candela (cd) |
You are probably already familiar with some derived quantities that can be formed from the base quantities in Table \(\PageIndex{1}\). For example, the geometric concept of area is always calculated as the product of two lengths. Thus, area is a derived quantity that can be expressed in terms of SI base units using square meters (m x m = m2). Similarly, volume is a derived quantity that can be expressed in cubic meters (m3). Speed is length per time; so in terms of SI base units, we could measure it in meters per second (m/s). Volume mass density (or just density) is mass per volume, which is expressed in terms of SI base units such as kilograms per cubic meter (kg/m3). Angles can also be thought of as derived quantities because they can be defined as the ratio of the arc length subtended by two radii of a circle to the radius of the circle. This is how the radian is defined. Depending on your background and interests, you may be able to come up with other derived quantities, such as the mass flow rate (kg/s) or volume flow rate (m3/s) of a fluid, electric charge (A • s), mass flux density [kg/(m2 • s)], and so on. We will see many more examples throughout this text. For now, the point is that every physical quantity can be derived from the seven base quantities in Table \(\PageIndex{1}\), and the units of every physical quantity can be derived from the seven SI base units.
For the most part, we use SI units in this text. Non-SI units are used in a few applications in which they are in very common use, such as the measurement of temperature in degrees Celsius (°C), the measurement of fluid volume in liters (L), and the measurement of energies of elementary particles in electron-volts (eV). Whenever non-SI units are discussed, they are tied to SI units through conversions. For example, 1 L is 10−3 m3.
Check out a comprehensive source of information on SI units at the National Institute of Standards and Technology (NIST) Reference on Constants, Units, and Uncertainty.
Units of Time, Length, and Mass: The Second, Meter, and Kilogram
The initial chapters in this textmap are concerned with mechanics, fluids, and waves. In these subjects all pertinent physical quantities can be expressed in terms of the base units of length, mass, and time. Therefore, we now turn to a discussion of these three base units, leaving discussion of the others until they are needed later.
The Second
The SI unit for time, the second (abbreviated s), has a long history. For many years it was defined as 1/86,400 of a mean solar day. More recently, a new standard was adopted to gain greater accuracy and to define the second in terms of a nonvarying or constant physical phenomenon (because the solar day is getting longer as a result of the very gradual slowing of Earth’s rotation). Cesium atoms can be made to vibrate in a very steady way, and these vibrations can be readily observed and counted. In 1967, the second was redefined as the time required for 9,192,631,770 of these vibrations to occur (Figure \(\PageIndex{2}\)). Note that this may seem like more precision than you would ever need, but it isn’t—GPSs rely on the precision of atomic clocks to be able to give you turn-by-turn directions on the surface of Earth, far from the satellites broadcasting their location.
The Meter
The SI unit for length is the meter (abbreviated m); its definition has also changed over time to become more precise. The meter was first defined in 1791 as 1/10,000,000 of the distance from the equator to the North Pole. This measurement was improved in 1889 by redefining the meter to be the distance between two engraved lines on a platinum–iridium bar now kept near Paris. By 1960, it had become possible to define the meter even more accurately in terms of the wavelength of light, so it was again redefined as 1,650,763.73 wavelengths of orange light emitted by krypton atoms. In 1983, the meter was given its current definition (in part for greater accuracy) as the distance light travels in a vacuum in 1/299,792,458 of a second (Figure \(\PageIndex{3}\)). This change came after knowing the speed of light to be exactly 299,792,458 m/s. The length of the meter will change if the speed of light is someday measured with greater accuracy.
The Kilogram
The SI unit for mass is the kilogram (abbreviated kg); From 1795–2018 it was defined to be the mass of a platinum–iridium cylinder kept with the old meter standard at the International Bureau of Weights and Measures near Paris. However, this cylinder has lost roughly 50 micrograms since it was created. Because this is the standard, this has shifted how we defined a kilogram. Therefore, a new definition was adopted in May 2019 based on the Planck constant and other constants which will never change in value. We will study Planck’s constant in quantum mechanics, which is an area of physics that describes how the smallest pieces of the universe work. The kilogram is measured on a Kibble balance (see \(\PageIndex{4}\)). When a weight is placed on a Kibble balance, an electrical current is produced that is proportional to Planck’s constant. Since Planck’s constant is defined, the exact current measurements in the balance define the kilogram.

Metric Prefixes
SI units are part of the metric system, which is convenient for scientific and engineering calculations because the units are categorized by factors of 10. Table \(\PageIndex{1}\) lists the metric prefixes and symbols used to denote various factors of 10 in SI units. For example, a centimeter is one-hundredth of a meter (in symbols, 1 cm = 10–2 m) and a kilometer is a thousand meters (1 km = 103 m). Similarly, a megagram is a million grams (1 Mg = 106 g), a nanosecond is a billionth of a second (1 ns = 10–9 s), and a terameter is a trillion meters (1 Tm = 1012 m).
| Prefix | Symbol | Meaning | Prefix | Symbol | Meaning |
|---|---|---|---|---|---|
| yotta- | Y | 1024 | yocto- | Y | 10-24 |
| zetta- | Z | 1021 | zepto- | Z | 10-21 |
| exa- | E | 1018 | atto- | E | 10-18 |
| peta- | P | 1015 | femto- | P | 10-15 |
| tera- | T | 1012 | pico- | T | 10-12 |
| giga- | G | 109 | nano- | G | 10-9 |
| mega- | M | 106 | micro- | M | 10-6 |
| kilo- | k | 103 | milli- | k | 10-3 |
| hecto- | h | 102 | centi- | h | 10-2 |
| deka- | da | 101 | deci- | da | 10-1 |
The only rule when using metric prefixes is that you cannot “double them up.” For example, if you have measurements in petameters (1 Pm = 1015 m), it is not proper to talk about megagigameters, although 106 x 109 = 1015. In practice, the only time this becomes a bit confusing is when discussing masses. As we have seen, the base SI unit of mass is the kilogram (kg), but metric prefixes need to be applied to the gram (g), because we are not allowed to “double-up” prefixes. Thus, a thousand kilograms (103 kg) is written as a megagram (1 Mg) since
\[10^{3}\; kg = 10^{3} \times 10^{3}\; g = 10^{6}\; g = 1\; Mg \ldotp\]
Incidentally, 103 kg is also called a metric ton, abbreviated t. This is one of the units outside the SI system considered acceptable for use with SI units.
As we see in the next section, metric systems have the advantage that conversions of units involve only powers of 10. There are 100 cm in 1 m, 1000 m in 1 km, and so on. In nonmetric systems, such as the English system of units, the relationships are not as simple—there are 12 in in 1 ft, 5280 ft in 1 mi, and so on.
Another advantage of metric systems is that the same unit can be used over extremely large ranges of values simply by scaling it with an appropriate metric prefix. The prefix is chosen by the order of magnitude of physical quantities commonly found in the task at hand. For example, distances in meters are suitable in construction, whereas distances in kilometers are appropriate for air travel, and nanometers are convenient in optical design. With the metric system there is no need to invent new units for particular applications. Instead, we rescale the units with which we are already familiar.
Restate the mass 1.93 x 1013 kg using a metric prefix such that the resulting numerical value is bigger than one but less than 1000.
Strategy
Since we are not allowed to “double-up” prefixes, we first need to restate the mass in grams by replacing the prefix symbol k with a factor of 103 (Table \(\PageIndex{2}\)). Then, we should see which two prefixes in Table \(\PageIndex{2}\) are closest to the resulting power of 10 when the number is written in scientific notation. We use whichever of these two prefixes gives us a number between one and 1000.
- Solution
-
Replacing the k in kilogram with a factor of 103, we find that
\[1.93 \times 10^{13}\; kg = 1.93 \times 10^{13} \times 10^{3}\; g = 1.93 \times 10^{16}\; g \ldotp \nonumber\]
From Table \(\PageIndex{2}\), we see that 1016 is between “peta-” (1015) and “exa-” (1018). If we use the “peta-” prefix, then we find that 1.93 × 1016 g = 1.93 × 101 Pg, since 16 = 1 + 15. Alternatively, if we use the “exa-” prefix we find that 1.93 x 1016 g = 1.93 x 10−2 Eg, since 16 = −2 + 18. Because the problem asks for the numerical value between one and 1000, we use the “peta-” prefix and the answer is 19.3 Pg.
Significance
It is easy to make silly arithmetic errors when switching from one prefix to another, so it is always a good idea to check that our final answer matches the number we started with. An easy way to do this is to put both numbers in scientific notation and count powers of 10, including the ones hidden in prefixes. If we did not make a mistake, the powers of 10 should match up. In this problem, we started with 1.93 x 1013 kg, so we have 13 + 3 = 16 powers of 10. Our final answer in scientific notation is 1.93 x 101 Pg, so we have 1 + 15 = 16 powers of 10. So, everything checks out.
If this mass arose from a calculation, we would also want to check to determine whether a mass this large makes any sense in the context of the problem. For this, Figure 1.4 might be helpful.
Restate 4.79 x 105 kg using a metric prefix such that the resulting number is bigger than one but less than 1000.
- Answer
-
Add texts here. Do not delete this text first.


