10.4: Cohesion and Adhesion
- Last updated
- Jan 14, 2019
- Save as PDF
- Page ID
- 16995

- Boundless
- Boundless
( \newcommand{\kernel}{\mathrm{null}\,}\)
learning objectives
- Explain the phenomena of surface tension and capillary action
Attractive forces between molecules of the same type are called cohesive forces. Liquids can, for example, be kept in open containers because cohesive forces hold the molecules together. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. In this section we examine effects of cohesive and adhesive forces in liquids.
Surface Tension
Surface tension is a contractive tendency of the surface of a liquid that allows it to resist an external force. It is shown, for example, in the floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects (e.g., water striders) to run on water’s surface. This property is caused by cohesion of similar molecules and is responsible for many of the behaviors of liquids.
The cohesive forces among liquid molecules are responsible for the phenomenon of surface tension, as shown in. In the bulk of the liquid, each molecule is pulled equally in every direction by neighboring liquid molecules, resulting in a net force of zero. The molecules at the surface do not have other molecules on all sides of them and therefore are pulled inwards. This creates some internal pressure and forces liquid surfaces to contract to the minimal area.
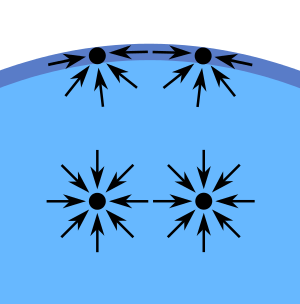
Diagram of Surface-Tension Forces: Diagram of the forces on molecules of a liquid
Surface tension has the unit of force per unit length, or of energy per unit area. The two units are equivalent. However, when we refer to energy per unit of area, we use the term surface energy, which is more general in that it applies to solids as well as liquids.
Capillary Action
Capillary action, or capillarity, is the ability of a liquid to flow in narrow spaces without the assistance of, and in opposition to, external forces like gravity. The effect can be seen in the drawing-up of liquids between the hairs of a paint-brush, in a thin tube, in porous materials such as paper, in some non-porous materials such as liquified carbon fiber, and in a cell. It occurs because of intermolecular attractive forces between the liquid and solid surrounding surfaces. If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and adhesive forces between the liquid and the container act to lift the liquid.
With some pairs of materials, such as mercury and glass (see ), the intermolecular forces within the liquid exceed those between the solid and the liquid, so a convex meniscus forms, and capillary action works in reverse.
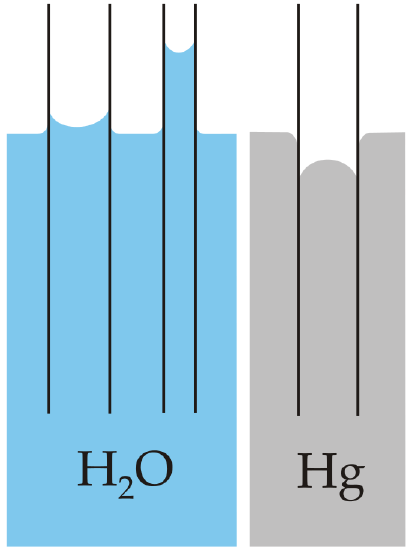
Capillarity: Capillary action of water compared to mercury, in each case with respect to glass
Key Points
- Attractive forces between molecules of the same type are called cohesive forces.
- Attractive forces between molecules of different types are called adhesive forces.
- Surface tension is a contractive tendency of the surface of a liquid that allows it to resist an external force.
- Capillary action, or capillarity, is the ability of a liquid to flow in narrow spaces without the assistance of, and in opposition to, external forces such as gravity.
Key Term
- Pressure: the amount of force that is applied over a given area divided by the size of that area
- intermolecular: from one molecule to another; between molecules
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax College, College Physics. September 17, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42197/latest/?collection=col11406/1.7. License: CC BY: Attribution
- OpenStax College, College Physics. September 17, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42197/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Surface tension. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Surface_tension. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. September 17, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42197/latest/?collection=col11406/1.7. License: CC BY: Attribution
- Capillary action. Provided by: Wikipedia. Located at: http://en.Wikipedia.org/wiki/Capillary_action. License: CC BY-SA: Attribution-ShareAlike
- Surface tension. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Surface_tension. License: CC BY-SA: Attribution-ShareAlike
- Adhesion. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Adhesion. License: CC BY-SA: Attribution-ShareAlike
- Cohesion (chemistry). Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Cohesion_(chemistry). License: CC BY-SA: Attribution-ShareAlike
- Surface tension. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Surface_tension. License: CC BY-SA: Attribution-ShareAlike
- intermolecular. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/intermolecular. License: CC BY-SA: Attribution-ShareAlike
- Pressure. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/Pressure. License: CC BY-SA: Attribution-ShareAlike
- File:Capillarity.svg - Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: en.Wikipedia.org/w/index.php?title=File:Capillarity.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikipedia. Located at: en.Wikipedia.org/w/index.php?title=File:Wassermolek%C3%BCleInTr%C3%B6pfchen.svg&page=1. License: Public Domain: No Known Copyright

