learning objectives
- Explain relationship between nuclear radius, nuclear density, and nuclear size.
Nuclear size is defined by nuclear radius, also called rms charge radius. It can be measured by the scattering of electrons by the nucleus and also inferred from the effects of finite nuclear size on electron energy levels as measured in atomic spectra.
The problem of defining a radius for the atomic nucleus is similar to the problem of atomic radius, in that neither atoms nor their nuclei have definite boundaries. However, the nucleus can be modelled as a sphere of positive charge for the interpretation of electron scattering experiments: because there is no definite boundary to the nucleus, the electrons “see” a range of cross-sections, for which a mean can be taken. The qualification of “rms” (for “root mean square”) arises because it is the nuclear cross-section, proportional to the square of the radius, which is determining for electron scattering.
The first estimate of a nuclear charge radius was made by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester, UK. The famous Rutherford gold foil experiment involved the scattering of α-particles by gold foil, with some of the particles being scattered through angles of more than 90°, that is coming back to the same side of the foil as the α-source, as shown in Figure 1. Rutherford was able to put an upper limit on the radius of the gold nucleus of 34 femtometers (fm).
Later studies found an empirical relation between the charge radius and the mass number, \(\mathrm{A}\), for heavier nuclei \((\mathrm { A } > 20 ): \mathrm { R } \approx \mathrm { r } \cdot \mathrm { A } ^ { 1 / 3 }\) where \(\mathrm{r}\) is an empirical constant of 1.2–1.5 fm. This gives a charge radius for the gold nucleus (A=197A=197) of about 7.5 fm.
Nuclear density is the density of the nucleus of an atom, averaging about \(4 \cdot 10 ^ { 17 } \mathrm { kg } / \mathrm { m } ^ { 3 }\). The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus:
\[\mathrm { n } = \dfrac { \mathrm { A } } { \frac { 4 } { 3 } \pi \mathrm { R } ^ { 3 } }\]
Nuclear Stability
The stability of an atom depends on the ratio and number of protons and neutrons, which may represent closed and filled quantum shells.
learning objectives
- Explain the relationship between the stability of an atom and its atomic structure.
The stability of an atom depends on the ratio of its protons to its neutrons, as well as on whether it contains a “magic number” of neutrons or protons that would represent closed and filled quantum shells. These quantum shells correspond to energy levels within the shell model of the nucleus. Filled shells, such as the filled shell of 50 protons in the element tin, confers unusual stability on the nuclide. Of the 254 known stable nuclides, only four have both an odd number of protons and an odd number of neutrons:
- hydrogen-2 (deuterium)
- lithium-6
- boron-10
- nitrogen-14
Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life greater than a billion years:
- potassium-40
- vanadium-50
- lanthanum-138
- tantalum-180m
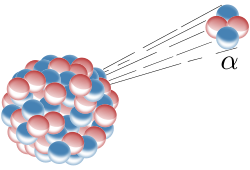
Alpha Decay: Alpha decay is one type of radioactive decay. An atomic nucleus emits an alpha particle and thereby transforms (“decays”) into an atom with a mass number smaller by four and an atomic number smaller by two. Many other types of decay are possible.
Most odd-odd nuclei are highly unstable with respect to beta decay because the decay products are even-even and therefore more strongly bound, due to nuclear pairing effects.
An atom with an unstable nucleus, called a radionuclide, is characterized by excess energy available either for a newly created radiation particle within the nucleus or via internal conversion. During this process, the radionuclide is said to undergo radioactive decay. Radioactive decay results in the emission of gamma rays and/or subatomic particles such as alpha or beta particles, as shown in. These emissions constitute ionizing radiation. Radionuclides occur naturally but can also be produced artificially.
All elements form a number of radionuclides, although the half-lives of many are so short that they are not observed in nature. Even the lightest element, hydrogen, has a well-known radioisotope: tritium. The heaviest elements (heavier than bismuth) exist only as radionuclides. For every chemical element, many radioisotopes that do not occur in nature (due to short half-lives or the lack of a natural production source) have been produced artificially.
Binding Energy and Nuclear Forces
Nuclear force is the force that is responsible for binding of protons and neutrons into atomic nuclei.
learning objectives
- Explain how nuclear force varies with distance.
The nuclear force is the force between two or more component parts of an atomic nuclei. The component parts are neutrons and protons, which collectively are called nucleons. Nuclear force is responsible for the binding of protons and neutrons into atomic nuclei.
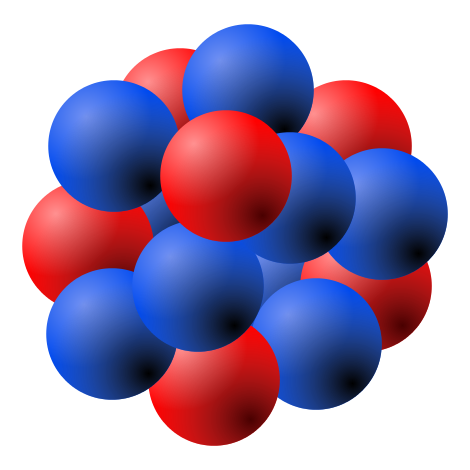
Drawing of Atomic Nucleus: A model of the atomic nucleus showing it as a compact bundle of the two types of nucleons: protons (red) and neutrons (blue).
To disassemble a nucleus into unbound protons and neutrons would require working against the nuclear force. Conversely, energy is released when a nucleus is created from free nucleons or other nuclei—known as the nuclear binding energy. The binding energy of nuclei is always a positive number, since all nuclei require net energy to separate into individual protons and neutrons. Because of mass-energy equivalence (i.e., Einstein’s famous formula \(\mathrm { E } = \mathrm { mc } ^ { 2 }\)), releasing this energy causes the mass of the nucleus to be lower than the total mass of the individual nucleons (leading to “mass deficit”). Binding energy is the energy used in nuclear power plants and nuclear weapons.
The nuclear force is powerfully attractive between nucleons at distances of about 1 femtometer (fm) between their centers, but rapidly decreases to relative insignificance at distances beyond about 2.5 fm. At very short distances (less than 0.7 fm) it becomes repulsive; it is responsible for the physical size of nuclei since the nucleons can come no closer than the force allows.
The nuclear force is now understood as a residual effect of an even more powerful “strong force” or strong interaction. It is the attractive force that binds together particles known as quarks (to form the nucleons themselves). This more powerful force is mediated by particles called gluons. Gluons hold quarks together with a force like that of an electric charge (but of far greater power).
The nuclear forces arising between nucleons are now seen as analogous to the forces in chemistry between neutral atoms or molecules (called London forces). Such forces between atoms are much weaker than the attractive electrical forces that hold together the atoms themselves (i.e., that bind electrons to the nucleus), and their range between atoms is shorter because they arise from a small separation of charges inside the neutral atom.
Similarly, even though nucleons are made of quarks in combinations which cancel most gluon forces (they are “color neutral”), some combinations of quarks and gluons leak away from nucleons in the form of short-range nuclear force fields that extend from one nucleon to another nucleon in close proximity. These nuclear forces are very weak compared to direct gluon forces (“color forces” or “strong forces”) inside nucleons, and the nuclear forces extend over only a few nuclear diameters, falling exponentially with distance. Nevertheless, they are strong enough to bind neutrons and protons over short distances, as well as overcome the electrical repulsion between protons in the nucleus. Like London forces, nuclear forces also stop being attractive, and become repulsive when nucleons are brought too close together.
Key Points
- The first estimate of a nuclear charge radius was made by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford, in the gold foil experiment that involved the scattering of α-particles by gold foil, as shown in Figure 1.
- An empirical relation exists between the charge radius and the mass number, \(\mathrm{A}\), for heavier nuclei (\(\mathrm{A>20}\)): where \(\mathrm{r}\) is an empirical constant of 1.2–1.5 fm.
- The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus: \(\mathrm { n } = \frac { \mathrm { A } } { \frac { 4 } { 3 } \pi \mathrm { R } ^ { 3 } }\)
- Most odd-odd nuclei are highly unstable with respect to beta decay because the decay products are even-even and therefore more strongly bound, due to nuclear pairing effects.
- An atom with an unstable nucleus is characterized by excess energy available either for a newly created radiation particle within the nucleus or via internal conversion.
- All elements form a number of radionuclides, although the half-lives of many are so short that they are not observed in nature.
- The nuclear force is powerfully attractive at distances of about 1 femtometer (fm), rapidly decreases to insignificance at distances beyond about 2.5 fm, and becomes repulsive at very short distances less than 0.7 fm.
- The nuclear force is a residual effect of the a strong interaction that binds together particles called quarks into nucleons.
- The binding energy of nuclei is always a positive number while the mass of an atom ‘s nucleus is always less than the sum of the individual masses of the constituent protons and neutrons when separated.
Key Terms
- α-particle: two protons and two neutrons bound together into a particle identical to a helium nucleus
- atomic spectra: emission or absorption lines formed when an electron makes a transition from one energy level of an atom to another
- nucleus: the massive, positively charged central part of an atom, made up of protons and neutrons
- nuclide: A nuclide (from “nucleus”) is an atomic species characterized by the specific constitution of its nucleus — i.e., by its number of protons (\(\mathrm{Z}\)), its number of neutrons (\(\mathrm{N}\)), and its nuclear energy state.
- radionuclide: A radionuclide is an atom with an unstable nucleus, characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or via internal conversion.
- radioactive decay: any of several processes by which unstable nuclei emit subatomic particles and/or ionizing radiation and disintegrate into one or more smaller nuclei
- nucleus: the massive, positively charged central part of an atom, made up of protons and neutrons
- quark: In the Standard Model, an elementary subatomic particle that forms matter. Quarks are never found alone in nature, but combine to form hadrons, such as protons and neutrons.
- gluon: A massless gauge boson that binds quarks together to form baryons, mesons and other hadrons; it is associated with the strong nuclear force.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Nuclear size. Provided by: Wikipedia. Located at: http://en.Wikipedia.org/wiki/Nuclear_size. License: CC BY-SA: Attribution-ShareAlike
- Nuclear density. Provided by: Wikipedia. Located at: http://en.Wikipedia.org/wiki/Nuclear_density. License: CC BY-SA: Attribution-ShareAlike
- Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/?-particle. License: CC BY-SA: Attribution-ShareAlike
- nucleus. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/nucleus. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/de...atomic-spectra. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/wikipedi...esults.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Radioactive decay. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radioactive_decay. License: CC BY-SA: Attribution-ShareAlike
- Unstable isotope. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Unstable_isotope. License: CC BY-SA: Attribution-ShareAlike
- nuclide. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/nuclide. License: CC BY-SA: Attribution-ShareAlike
- radionuclide. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/radionuclide. License: CC BY-SA: Attribution-ShareAlike
- radioactive decay. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radioactive_decay. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/wikipedi...esults.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Binding energy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Binding_energy. License: CC BY-SA: Attribution-ShareAlike
- Nuclear binding energy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_binding_energy. License: CC BY-SA: Attribution-ShareAlike
- Nuclear force. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_force. License: CC BY-SA: Attribution-ShareAlike
- nucleus. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/nucleus. License: CC BY-SA: Attribution-ShareAlike
- gluon. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/gluon. License: CC BY-SA: Attribution-ShareAlike
- quark. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/quark. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/wikipedi...esults.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/wikipedi..._Decay.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...rawing.svg.png. License: CC BY-SA: Attribution-ShareAlike



