18.6: Ionic Crystal Structure
- Page ID
- 102004
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Why are crystals appealing?
Crystals are found everywhere that chemical deposits are located. Ruby crystals are extremely valuable, both because of ruby's beauty and its utility in equipment such as lasers. Some claim that crystals have magical qualities. For others, the "magic" is in the regular structure of the crystal, as the cations and anions line up in a regular order.
Ionic Crystal Structure
Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. However, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. In order to minimize the potential energy of the system, ionic compounds take on the form of an extended three-dimensional array of alternating cations and anions. This maximizes the attractive forces between the oppositely charged ions. The figure below shows two different ways of representing the ionic crystal lattice. A ball and stick model makes it easier to see how individual ions are oriented with respect to one another. A space filling diagram is a more accurate representation of how the ions pack together in the crystal.
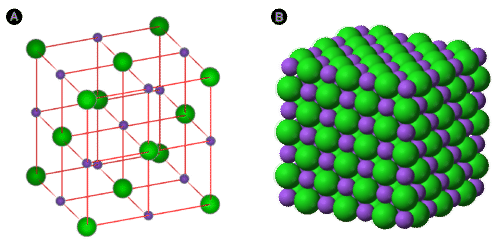 Figure \(\PageIndex{2}\): Two models of a sodium chloride crystal are shown. The purple spheres represent the \(\ce{Na^+}\) ions, while the green spheres represent the \(\ce{Cl^-}\) ions. (A) In an expanded view, the distances between ions are exaggerated, more easily showing the coordination numbers of each ion. (B) In a space filling model, the electron clouds of the ions are in contact with each other. (Public Domain; (A) Eloy; (B) Ben Mills (Wikimedia: Benjah-bmm27) via (A) http://commons.wikimedia.org/wiki/File:NaCl-estructura_cristalina.svg; (B) http://commons.wikimedia.org/wiki/File:Sodium-chloride-3D-ionic.png)
Figure \(\PageIndex{2}\): Two models of a sodium chloride crystal are shown. The purple spheres represent the \(\ce{Na^+}\) ions, while the green spheres represent the \(\ce{Cl^-}\) ions. (A) In an expanded view, the distances between ions are exaggerated, more easily showing the coordination numbers of each ion. (B) In a space filling model, the electron clouds of the ions are in contact with each other. (Public Domain; (A) Eloy; (B) Ben Mills (Wikimedia: Benjah-bmm27) via (A) http://commons.wikimedia.org/wiki/File:NaCl-estructura_cristalina.svg; (B) http://commons.wikimedia.org/wiki/File:Sodium-chloride-3D-ionic.png)
Naturally occurring sodium chloride (halite) does not look at first glance like the neat diagrams shown above. It is only when we use modern techniques to analyze the crystal structure at the atomic level that we can see the true regularity of the organized ions.
 Figure \(\PageIndex{3}\): Halite crystals. (Public Domain; Ingo Wölbern (Wikimedia: Iwoelbern) via Wikipedia)
Figure \(\PageIndex{3}\): Halite crystals. (Public Domain; Ingo Wölbern (Wikimedia: Iwoelbern) via Wikipedia)
Summary
- Ionic compounds take on the form of extended three-dimensional arrays of cations and anions.
- The arrangement maximizes the attractive force between oppositely-charged ions.
Review
- Why do ionic compounds form crystal structure instead of discrete molecules?
- Ions arrange themselves in three-dimensional array to maximize what type of force
- Which of the following would best describe how ions in an ionic compound arrange themselves?
- randomly
- an orderly pattern

