19.14: Hybrid Orbitals - sp and sp²
( \newcommand{\kernel}{\mathrm{null}\,}\)
 Figure 19.14.1 (Public Domain; Frank Dicksee via Wikipedia)
Figure 19.14.1 (Public Domain; Frank Dicksee via Wikipedia)
How do you open the closed circle?
Romeo and Juliet were two of the greatest-known fictional lovers of all time. Their embrace allowed no other person to be a part of it—they only wanted to be with each other. It took outside intervention to get them away from one another. Paired electrons are similar to Romeo and Juliet. They do not bond covalently until they are unpaired; then, they can become a part of a larger chemical structure.
sp Hybridization
A beryllium hydride (BeH2) molecule is predicted to be linear by VSEPR. The beryllium atom contains all paired electrons and so must also undergo hybridization. One of the 2s electrons is first promoted to the empty 2px orbital (see figure below).
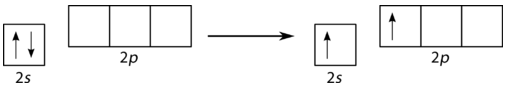 Figure 19.14.2: Promotion of Be 2s electron. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
Figure 19.14.2: Promotion of Be 2s electron. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
Now the hybridization takes place only with occupied orbitals, and the result is a pair of sp hybrid orbitals. The two remaining p orbitals (py and pz) do not hybridize and remain unoccupied (see figure below).
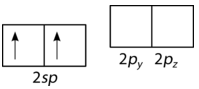 Figure 19.14.3: Be hybrid orbitals. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
Figure 19.14.3: Be hybrid orbitals. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
The geometry of the sp hybrid orbitals is linear, with the lobes of the orbitals pointing in opposite directions along one axis, arbitrarily defined as the x-axis (see figure below). Each can bond with a 1s orbital from a hydrogen atom to form the linear BeH2 molecule.
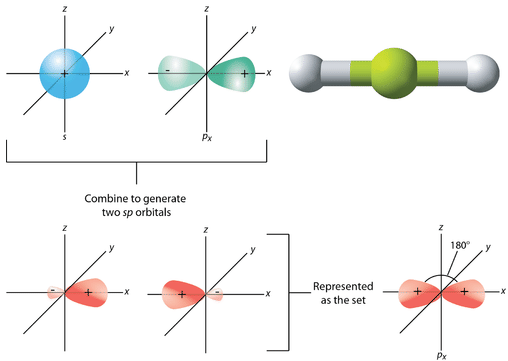 Figure 19.14.4: The process of sp hybridization is the mixing of an s orbital with a single p orbital (the px orbital by convention), to form a set of two sp hybrids. The two lobes of the sp hybrids point opposite one another to produce a linear molecule. (CC BY-NC 3.0 (3D molecule available under public domain; Jodi So, using 3D molecule by Ben Mills (User: Benjah-bmm27/Wikimedia Commons via CK-12 Foundation; 3D molecule: http://commons.wikimedia.org/wiki/File:Beryllium-hydride-molecule-IR-3D-balls.png))
Figure 19.14.4: The process of sp hybridization is the mixing of an s orbital with a single p orbital (the px orbital by convention), to form a set of two sp hybrids. The two lobes of the sp hybrids point opposite one another to produce a linear molecule. (CC BY-NC 3.0 (3D molecule available under public domain; Jodi So, using 3D molecule by Ben Mills (User: Benjah-bmm27/Wikimedia Commons via CK-12 Foundation; 3D molecule: http://commons.wikimedia.org/wiki/File:Beryllium-hydride-molecule-IR-3D-balls.png))
Other molecules whose electron domain geometry is linear and for whom hybridization is necessary also form sp hybrid orbitals. Examples include CO2 and C2H2, which will be discussed in further detail later.
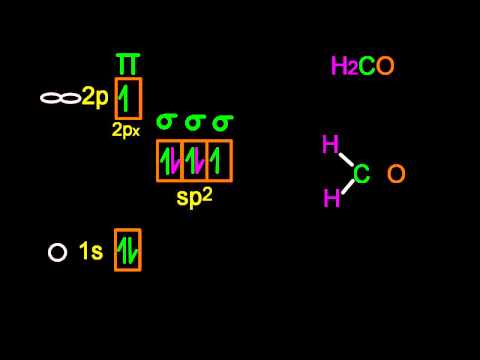
sp² Hybridization
Boron trifluoride (BF3) is predicted to have a trigonal planar geometry by VSEPR. First, a paired 2s electron is promoted to the empty 2py orbital (see figure below).
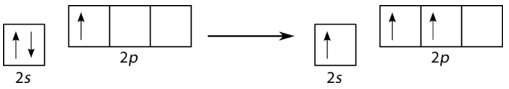 Figure 19.14.5: Promotion of 2s electron. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
Figure 19.14.5: Promotion of 2s electron. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
This is followed by hybridization of the three occupied orbitals to form a set of three sp2 hybrids, leaving the 2pz orbital unhybridized (see figure below).
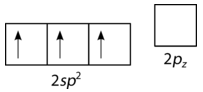 Figure 19.14.6: Formation of sp2 orbital. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
Figure 19.14.6: Formation of sp2 orbital. (CC BY-NC 3.0; Joy Sheng via CK-12 Foundation)
The geometry of the sp2 hybrid orbitals is trigonal planar, with the lobes of the orbitals pointing towards the corners of a triangle (see figure below). The angle between any two of the hybrid orbital lobes is 120o. Each can bond with a 2p orbital from a fluorine atom to form the trigonal planar BF3 molecule.
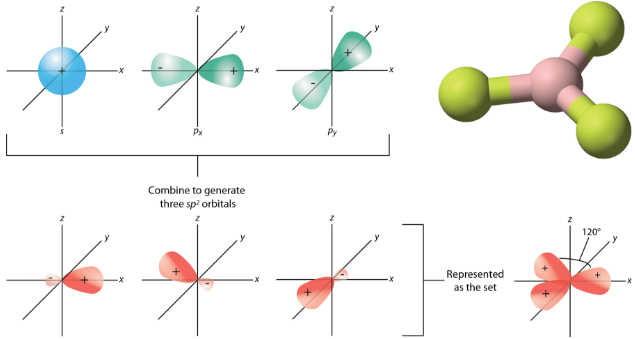 Figure 19.14.7: The process of sp2 hybridization is the mixing of an s orbital with a set of two p orbitals (px and py) to form a set of three sp2 hybrid orbitals. Each large lobe of the hybrid orbital points to one corner of a planar triangle. (CC-BY-NC 3.0 (3D molecule available under the public domain; CK-12 Foundation - Jodi So, using 3D molecular structure by Ben Mills (Wikimedia: Benjah-bmm27) via 3D molecule: http://commons.wikimedia.org/wiki/File:Boron-trifluoride-3D-balls.png))
Figure 19.14.7: The process of sp2 hybridization is the mixing of an s orbital with a set of two p orbitals (px and py) to form a set of three sp2 hybrid orbitals. Each large lobe of the hybrid orbital points to one corner of a planar triangle. (CC-BY-NC 3.0 (3D molecule available under the public domain; CK-12 Foundation - Jodi So, using 3D molecular structure by Ben Mills (Wikimedia: Benjah-bmm27) via 3D molecule: http://commons.wikimedia.org/wiki/File:Boron-trifluoride-3D-balls.png))
Other molecules with a trigonal planar electron domain geometry form sp2 hybrid orbitals. Ozone (O3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen makes the molecular geometry bent. The hybridization of the central O atom of ozone is sp2.
Summary
- Paired electrons can be hybridized and then participate in covalent bonding.
Review
- Does the ground state beryllium atom contain any unpaired electrons?
- Why does one 2s electron in Be get promoted to a 2p orbital?
- What is the geometry of the two sp orbitals?