10.8: Powering the Body
- Page ID
- 17797
Chemical Potential Energy
We have learned that when you jump, bend a paper clip, or lift an object you transfer energy to the objects, but where did that energy come from and what form was it in before you did work to transfer it into kinetic energy, potential energy, or thermal energy? The energy was stored as chemical potential energy in specific bonds within molecules in your muscle cells, specifically ATP molecules. We should note that chemical potential energy is stored in the separation electrically charged particles that make up atoms, analogous to the way gravitational potential energy is stored in the separation of masses. Therefore chemical potential energy is actually just a form of electrical potential energy, but we will not cover the details of electrical potential energy in this textbook.
The Biological Energy Cascade
The chemical potential energy stored in bonds within ATP is released to do work on muscle fibers (Actin and Myosin) and then work must be done to reform those bonds. That work is done during the ATP cycle shown in the following animation:
The energy to power the ATP cycle is transferred out of chemical potential energy in glucose molecules during cellular respiration. Those glucose molecules entered your body through the food you ate, and ultimately, the chemical potential energy they stored was transferred from electromagnetic energy in sunlight by plants via photosynthesis. [1]. To learn more about these processes consider taking courses in human anatomy and physiology, general biology, cell biology, molecular biology, and biochemistry.
Everyday Example: No work and all heat
Hold an object up in the air. Keep holding. Do you eventually get tired? Why? You are certainly applying a force, but the object hasn’t moved any distance, so it would appear that you really done anywork. Why should you get tired when you aren’t doing any work? The animation in the previous video provides the answer, you haven’t done any useful work, but you have work on a microscopic scale to transfer chemical potential energy to thermal energy. The ATP cycle occurs repeatedly just maintain muscle tension, even if the muscle does not actually move a noticeable distance. The ATP cycle continues to use up chemical potential energy even if you aren’t doing any useful work. Where does that energy go? Into thermal energy. If you hold the object long enough, you might even begin to sweat! If using stored energy without doing useful work seems pretty inefficient, you’re right. In fact the efficiency of the body in such a situation is zero! The next chapter will discuss the efficiency in greater detail.
Elastic Potential Energy in the Body
There are biochemical limits on how quickly your body can break down ATP to release chemical potential energy, which limits the rate at which your body is able to do work, also known as power (P). For example, making a change in speed changes your kinetic energy, which requires work. Quick changes in speed require the work to be done in short time interval, which equates to a high power output. You can apply some strategies to overcome biochemical power limitations in the short term by storing elastic potential energy in tissue tension for timed release. For example you can store elastic potential energy in your Achilles tendon when you squat down before jumping, then release that energy during the launch phase of a jump. Your body isn’t able to store this elastic energy potential energy for long, however, so it only provide short-term enhancements to power output.
Dr. Shelia Patek, Chair of the Biomechanics Division at the Society for Integrative and Comparative Biology, discovered that the mantis shrimp has doubled down on the elastic potential energy strategy by using a “structure in the arm that looks like a saddle or a Pringle chip. When the arm is cocked, this structure is compressed and acts like a spring, storing up even more energy. When the latch is released, the spring expands and provides extra push for the club, helping to accelerate it at up to 10,000 times the acceleration [caused by the] force of gravity on Earth [alone].”[2]
Quantifying Elastic Potential Energy
We often model particular tissues or other material as a springs, so elastic potential energy is sometimes used interchangeably with spring potential energy. The amount of elastic potential energy you can store in a spring can be calculated from the spring constant (k) and displacement 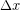 according to Hooke's Law:
according to Hooke's Law:
(1)
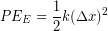
Everyday Example:
How much elastic potential energy is stored in the Achilles tendon during the crouch phase of a jump?
In the Modeling Tissues as Springs chapter of Unit 7 we estimated the typical spring constant of the Achilles tendon to be 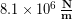 . During a jump the tendon may experience strain of more than 0.06, or 6 %. [3]We can use our equation for elastic potential energy above, but first we need to find the stretch distance corresponding to that strain value for a typical Achilles tendon length of 0.15 m. Starting with the strain equation:
. During a jump the tendon may experience strain of more than 0.06, or 6 %. [3]We can use our equation for elastic potential energy above, but first we need to find the stretch distance corresponding to that strain value for a typical Achilles tendon length of 0.15 m. Starting with the strain equation:
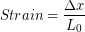
Rearranging for the stretch distance (displacement):
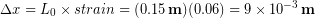
We can now calculate the elastic potential energy stored in the tendon during a jump:
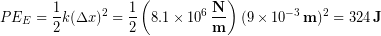
Using the equation for a change in gravitational potential energy we find out that the stored elastic potential energy is enough energy to launch a 65 kg person an additional 0.5 m into the air.
![]()
Storing elastic potential energy in tissues for timed release in parallel with muscle contraction can significantly increase the power output during a jump.
Reinforcement Exercises
A child’s toy uses a spring to launch small plastic balls into the air. If it takes 8 N of force to compress the spring 0.025 m, what is the spring constant? [Hint: Use Hooke's Law]
Now that you know the spring constant, what is the elastic potential energy stored in the spring when fully compressed to 0.5 m?
If the spring were used to launch a 0.006 kg plastic ball, how fast would the ball be moving (ignoring friction and air resistance)?
Power
We have seen that storage and time release of elastic potential energy can improve short term power output, or work done per unit time. The power for any energy conversion process can be calculated as:
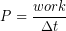
Power has units of J/s, also known as Watts (W). Another common unit for power is horsepower (hp). There are 746 W per hp.
Reinforcement Exercises
A 65 kg person starts at rest and then speeds up to 4 m/s. What is the change in kinetic energy of the person?
If this change in kinetic energy occurred over 3 s, what is the mechanical power output of the person?
Convert this power from units of Watts to horsepower.
The true rate at which the person uses chemical potential energy is actually much greater than this because the body is not 100 % efficient at converting chemical potential energy into kinetic energy. We will learn more about the efficiency of the body in the next few chapters.
Everyday Examples: Power Plants
Power plants convert energy from one form to another. The most common type convert chemical potential energy into thermal energy via combustion, and then convert thermal energy stored in steam, and then into kinetic energy via turbines. A large power plant might have an output of 500 million Watts, or 500 MW.
When you receive a power bill in the U.S. the typical unit you are billed for is kiloWatt-hours (kW-hr). These units can be confusing because we see Watt and think power, but this is actually a unit of energy. This makes sense because you should be billed based on the energy you used, but let's break down the confusing units.
Power is energy divided by time, so a power multiplied by a time is an energy:

The issue here is we a mixing time units, specifically hours and the seconds that are inside Watts. The reason might be that customers can better relate to kW-hr than joules when thinking about their energy usage. For example, leaving ten 100 W light bulbs on for one hour would be one kW-hr of energy:
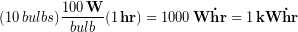
- OpenStax College, Biology. OpenStax CNX. Feb 25, 2016 [1]http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@9.92. ↵
- "The Mantis Shrimp Has the World’s Fastest Punch" by Ed Yong, Science and Innovation, National Geographic↵
- "Achilles tendon material properties are greater in the jump leg of jumping athletes" by Bayliss, A. J., Weatherholt, A. M., Crandall, T. T., Farmer, D. L., McConnell, J. C., Crossley, K. M., & Warden, S. J., National Library of Medicine, U.S. National Institutes of Heatlh↵




