8.13: Entropy and the Second Law of Thermodynamics- Disorder and the Unavailability of Energy
( \newcommand{\kernel}{\mathrm{null}\,}\)
Learning Objectives
- Define entropy and calculate the increase of entropy in a system with reversible and irreversible processes.
- Calculate the increasing disorder of a system.

There is yet another way of expressing the second law of thermodynamics. This version relates to a concept called entropy. By examining it, we shall see that the directions associated with the second law—heat transfer from hot to cold, for example—are related to the tendency in nature for systems to become disordered and for less energy to be available for use as work. The entropy of a system can in fact be shown to be a measure of its disorder and of the unavailability of energy to do work.
MAKING CONNECTIONS: ENTROPY, ENERGY, AND WORK
Recall that the simple definition of energy is the ability to do work. Entropy is a measure of how much energy is not available to do work. Although all forms of energy are interconvertible, and all can be used to do work, it is not always possible, even in principle, to convert the entire available energy into work. That unavailable energy is of interest in thermodynamics, because the field of thermodynamics arose from efforts to convert heat to work.
We can see how entropy is defined by recalling our discussion of the Carnot engine. We noted that for a Carnot cycle, and hence for any reversible processes, Qc/Qh=Tc/Th. Rearranging terms yields
QcTc=QhTh
for any reversible process. Qc and Qh are absolute values of the heat transfer at temperatures Tc and Th, respectively. This ratio of Q/T is defined to be the change in entropy ΔS for a reversible process,
ΔS=(QT)rev,
where Q is the heat transfer, which is positive for heat transfer into and negative for heat transfer out of, and T is the absolute temperature at which the reversible process takes place. The SI unit for entropy is joules per kelvin (J/K). If temperature changes during the process, then it is usually a good approximation (for small changes in temperature) to take T to be the average temperature, avoiding the need to use integral calculus to find ΔS.
The definition of ΔS is strictly valid only for reversible processes, such as used in a Carnot engine. However, we can find ΔS precisely even for real, irreversible processes. The reason is that the entropy S of a system, like internal energy U, depends only on the state of the system and not how it reached that condition. Entropy is a property of state. Thus the change in entropy ΔS of a system between state 1 and state 2 is the same no matter how the change occurs. We just need to find or imagine a reversible process that takes us from state 1 to state 2 and calculate ΔS for that process. That will be the change in entropy for any process going from state 1 to state 2. (See Figure 8.13.2.)
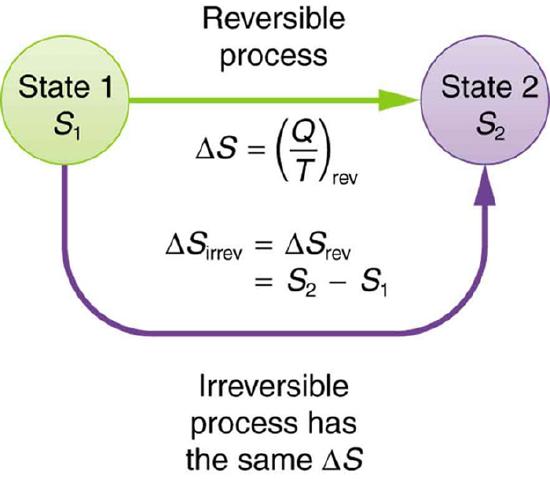
Now let us take a look at the change in entropy of a Carnot engine and its heat reservoirs for one full cycle. The hot reservoir has a loss of entropy ΔSh=−Qh/Th, because heat transfer occurs out of it (remember that when heat transfers out, then Q has a negative sign). The cold reservoir has a gain of entropy ΔSc=Qc/Tc, because heat transfer occurs into it. (We assume the reservoirs are sufficiently large that their temperatures are constant.) So the total change in entropy is
ΔStot =ΔSh+ΔSc
Thus, since we know that Qh/Th=Qc/Tc for a Carnot engine,
ΔStot=−QhTh+QcTc=0.
This result, which has general validity, means that the total change in entropy for a system in any reversible process is zero.
The entropy of various parts of the system may change, but the total change is zero. Furthermore, the system does not affect the entropy of its surroundings, since heat transfer between them does not occur. Thus the reversible process changes neither the total entropy of the system nor the entropy of its surroundings. Sometimes this is stated as follows: Reversible processes do not affect the total entropy of the universe. Real processes are not reversible, though, and they do change total entropy. We can, however, use hypothetical reversible processes to determine the value of entropy in real, irreversible processes. The following example illustrates this point.
Example 8.13.1: Entropy Increases in an Irreversible (Real) Process
Spontaneous heat transfer from hot to cold is an irreversible process. Calculate the total change in entropy if 4000 J of heat transfer occurs from a hot reservoir at Th=600 K (327∘C) to a cold reservoir at Tc=250 K (−23∘C), assuming there is no temperature change in either reservoir. (See Figure 8.13.3.)
Strategy
How can we calculate the change in entropy for an irreversible process when ΔStot=ΔSh+ΔSc is valid only for reversible processes? Remember that the total change in entropy of the hot and cold reservoirs will be the same whether a reversible or irreversible process is involved in heat transfer from hot to cold. So we can calculate the change in entropy of the hot reservoir for a hypothetical reversible process in which 4000 J of heat transfer occurs from it; then we do the same for a hypothetical reversible process in which 4000 J of heat transfer occurs to the cold reservoir. This produces the same changes in the hot and cold reservoirs that would occur if the heat transfer were allowed to occur irreversibly between them, and so it also produces the same changes in entropy.
Solution
We now calculate the two changes in entropy using ΔStot =ΔSh+ΔSc. First, for the heat transfer from the hot reservoir,
ΔSh=−QhTh=−4000 J600 K=−6.67 J/K.
And for the cold reservoir,
ΔSc=QcTc=4000 J250 K=16.0 J/K.
Thus the total is
ΔStot =ΔSh+ΔSc=(−6.67+16.0)J/K=9.33 J/K.
Discussion
There is an increase in entropy for the system of two heat reservoirs undergoing this irreversible heat transfer. We will see that this means there is a loss of ability to do work with this transferred energy. Entropy has increased, and energy has become unavailable to do work.
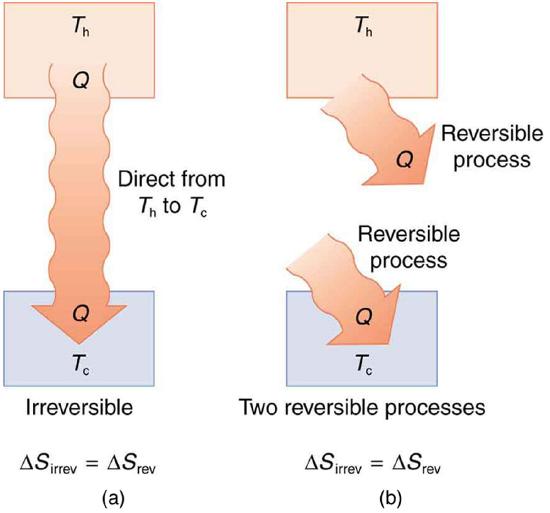
It is reasonable that entropy increases for heat transfer from hot to cold. Since the change in entropy is Q/T, there is a larger change at lower temperatures. The decrease in entropy of the hot object is therefore less than the increase in entropy of the cold object, producing an overall increase, just as in the previous example. This result is very general:
There is an increase in entropy for any system undergoing an irreversible process.
With respect to entropy, there are only two possibilities: entropy is constant for a reversible process, and it increases for an irreversible process. There is a fourth version of the second law of thermodynamics stated in terms of entropy:
The total entropy of a system either increases or remains constant in any process; it never decreases.
For example, heat transfer cannot occur spontaneously from cold to hot, because entropy would decrease.
Entropy is very different from energy. Entropy is not conserved but increases in all real processes. Reversible processes (such as in Carnot engines) are the processes in which the most heat transfer to work takes place and are also the ones that keep entropy constant. Thus we are led to make a connection between entropy and the availability of energy to do work.
Order to Disorder
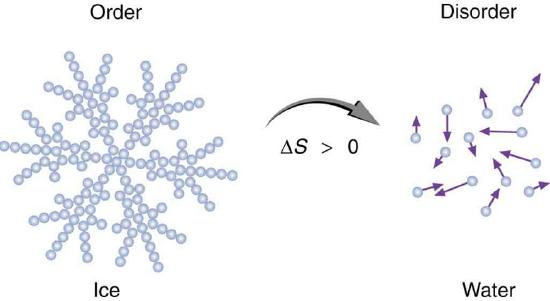
Entropy is related not only to the unavailability of energy to do work—it is also a measure of disorder. This notion was initially postulated by Ludwig Boltzmann in the 1800s. For example, melting a block of ice means taking a highly structured and orderly system of water molecules and converting it into a disorderly liquid in which molecules have no fixed positions. (See Figure 8.13.4.) There is a large increase in entropy in the process, as seen in the following example.
Example 8.13.2: Entropy Associated with Disorder
Find the increase in entropy of 1.00 kg of ice originally at 0∘C that is melted to form water at 0∘C.
Strategy
As before, the change in entropy can be calculated from the definition of ΔS once we find the energy Q needed to melt the ice.
Solution
The change in entropy is defined as:
ΔS=QT.
Here Q is the heat transfer necessary to melt 1.00 kg of ice and is given by
Q=mLf,
where m is the mass and Lf is the latent heat of fusion. Lf=334 kJ/kg for water, so that
Q=(1.00 kg)(334 kJ/kg)=3.34×105 J.
Now the change in entropy is positive, since heat transfer occurs into the ice to cause the phase change; thus,
ΔS=QT=3.34×105 JT.
T is the melting temperature of ice. That is, T=0∘C=273 K. So the change in entropy is
ΔS=3.34×105 J273 K=1.22×103 J/K.
Discussion
This is a significant increase in entropy accompanying an increase in disorder.
In another easily imagined example, suppose we mix equal masses of water originally at two different temperatures, say 20.0∘C and 40.0∘C. The result is water at an intermediate temperature of 30.0∘C. Three outcomes have resulted: entropy has increased, some energy has become unavailable to do work, and the system has become less orderly. Let us think about each of these results.
First, entropy has increased for the same reason that it did in the example above. Mixing the two bodies of water has the same effect as heat transfer from the hot one and the same heat transfer into the cold one. The mixing decreases the entropy of the hot water but increases the entropy of the cold water by a greater amount, producing an overall increase in entropy.
Second, once the two masses of water are mixed, there is only one temperature—you cannot run a heat engine with them. The energy that could have been used to run a heat engine is now unavailable to do work.
Third, the mixture is less orderly, or to use another term, less structured. Rather than having two masses at different temperatures and with different distributions of molecular speeds, we now have a single mass with a uniform temperature.
These three results—entropy, unavailability of energy, and disorder—are not only related but are in fact essentially equivalent.
Section Summary
- Entropy is the loss of energy available to do work.
- Another form of the second law of thermodynamics states that the total entropy of a system either increases or remains constant; it never decreases.
- Change of entropy is zero in a reversible process; it increases in an irreversible process.
- Entropy is also associated with the tendency toward disorder in an isolated system.
Glossary
- entropy
- a measurement of a system's disorder and its inability to do work in a system
- change in entropy
- the ratio of heat transfer to temperature Q/T
- second law of thermodynamics stated in terms of entropy
- the total entropy of a system either increases or remains constant; it never decreases


