3.6: Heat Capacities of an Ideal Gas
( \newcommand{\kernel}{\mathrm{null}\,}\)
By the end of this section, you will be able to:
- Define heat capacity of an ideal gas for a specific process
- Calculate the specific heat of an ideal gas for either an isobaric or isochoric process
- Explain the difference between the heat capacities of an ideal gas and a real gas
- Estimate the change in specific heat of a gas over temperature ranges
We learned about specific heat and molar heat capacity previously; however, we have not considered a process in which heat is added. We do that in this section. First, we examine a process where the system has a constant volume, then contrast it with a system at constant pressure and show how their specific heats are related.
Let’s start with looking at Figure \PageIndex{1}, which shows two vessels A and B, each containing 1 mol of the same type of ideal gas at a temperature T and a volume V. The only difference between the two vessels is that the piston at the top of A is fixed, whereas the one at the top of B is free to move against a constant external pressure p. We now consider what happens when the temperature of the gas in each vessel is slowly increased to T + dT with the addition of heat.
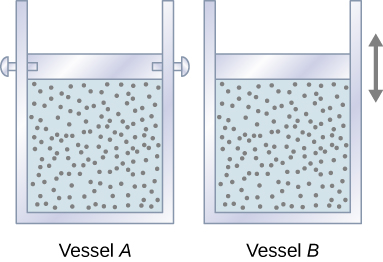
Since the piston of vessel A is fixed, the volume of the enclosed gas does not change. Consequently, the gas does no work, and we have from the first law
dE_{int} = dQ - dW = dQ. \nonumber
We represent the fact that the heat is exchanged at constant volume by writing
dQ = C_VndT, \nonumber where C_V is the molar heat capacity at constant volume of the gas. In addition, since dE_{int} = dQ for this particular process,
dE_{int} = C_VndT. \label{3.9}
We obtained this equation assuming the volume of the gas was fixed. However, internal energy is a state function that depends on only the temperature of an ideal gas. Therefore, dE_{int} = C_VndT gives the change in internal energy of an ideal gas for any process involving a temperature change dT.
When the gas in vessel B is heated, it expands against the movable piston and does work dW = pdV. In this case, the heat is added at constant pressure, and we write dQ = C_{p}ndT, \nonumber where C_p is the molar heat capacity at constant pressure of the gas. Furthermore, since the ideal gas expands against a constant pressure,
d(pV) = d(RnT) \nonumber becomes pdV = RndT. \nonumber
Finally, inserting the expressions for dQ and pdV into the first law, we obtain
dE_{int} = dQ - pdV = (C_{p}n - Rn)dT. \nonumber
We have found dE_{int} for both an isochoric and an isobaric process. Because the internal energy of an ideal gas depends only on the temperature, dE_{int} must be the same for both processes. Thus,
C_{V}ndT = (C_{p}n - Rn)dT, \nonumber
and
C_p = C_V + R. \label{eq50}
The derivation of Equation \ref{eq50} was based only on the ideal gas law. Consequently, this relationship is approximately valid for all dilute gases, whether monatomic like He, diatomic like O_2, or polyatomic like CO_2 or NH_3.
In the preceding chapter, we found the molar heat capacity of an ideal gas under constant volume to be
C_V = \dfrac{d}{2}R, \nonumber
where d is the number of degrees of freedom of a molecule in the system. Table \PageIndex{1} shows the molar heat capacities of some dilute ideal gases at room temperature. The heat capacities of real gases are somewhat higher than those predicted by the expressions of C_V and C_p given in Equation \ref{eq50}. This indicates that vibrational motion in polyatomic molecules is significant, even at room temperature. Nevertheless, the difference in the molar heat capacities, C_p - C_V, is very close to R, even for the polyatomic gases.
| C_p | C_V | C_p - C_V | ||
|---|---|---|---|---|
| Type of Molecule | Gas | (J/mol K) | (J/mol K) | (J/mol K) |
| Monatomic | Ideal | \frac{5}{2}R = 20.79 | \frac{3}{2}R = 12.47 | R = 8.31 |
| Diatomic | Ideal | \frac{7}{2}R = 29.10 | \frac{5}{2}R = 20.79 | R = 8.31 |
| Polyatomic | Ideal | 4R = 33.26 | 3R = 24.04 | R = 8.31 |
Glossary
- molar heat capacity at constant pressure
- quantifies the ratio of the amount of heat added removed to the temperature while measuring at constant pressure
- molar heat capacity at constant volume
- quantifies the ratio of the amount of heat added removed to the temperature while measuring at constant volume


