29.5: Multielectron Atoms
- Page ID
- 16240
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)learning objectives
- Describe atomic structure and shielding in multielectron atoms
Multielectron Atoms
Atoms with more than one electron, such as Helium (He) and Nitrogen (N), are referred to as multielectron atoms. Hydrogen is the only atom in the periodic table that has one electron in the orbitals under ground state.
In hydrogen-like atoms (those with only one electron), the net force on the electron is just as large as the electric attraction from the nucleus. However, when more electrons are involved, each electron (in the nn-shell) feels not only the electromagnetic attraction from the positive nucleus, but also repulsion forces from other electrons in shells from ‘1’ to ‘nn‘. This causes the net force on electrons in the outer electron shells to be significantly smaller in magnitude. Therefore, these electrons are not as strongly bonded to the nucleus as electrons closer to the nucleus. This phenomenon is often referred to as the Orbital Penetration Effect. The shielding theory also explains why valence shell electrons are more easily removed from the atom.
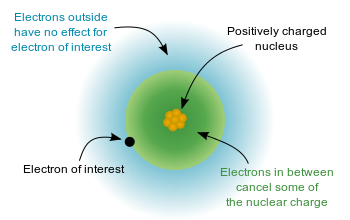
Electron Shielding Effect: A multielectron atom with inner electrons shielding outside electrons from the positively charged nucleus
The size of the shielding effect is difficult to calculate precisely due to effects from quantum mechanics. As an approximation, the effective nuclear charge on each electron can be estimated by: \(\mathrm{Z_{eff}=Z−σZ}_\text{eff} = \mathrm{Z} – \sigma \), where \(\mathrm{Z}\) is the number of protons in the nucleus and σ\sigma is the average number of electrons between the nucleus and the electron in question. σ\sigma can be found by using quantum chemistry and the Schrodinger equation or by using Slater’s empirical formula.
For example, consider a sodium cation, a fluorine anion, and a neutral neon atom. Each has 10 electrons, and the number of nonvalence electrons is two (10 total electrons minus eight valence electrons), but the effective nuclear charge varies because each has a different number of protons:
\[\mathrm { Z } _ { \mathrm { eff } } \mathrm { F } ^ { - } = 9 - 2 = 7 +\]
\[\mathrm { Z } _ { \mathrm { eff } } \mathrm { Ne } = 10 - 2 = 8 +\]
\[\mathrm { Z } _ { \mathrm { eff } } \mathrm { Na } ^ { + } = 11 - 2 = 9 +\]
As a consequence, the sodium cation has the largest effective nuclear charge and, therefore, the smallest atomic radius.
The Periodic Table
A periodic table is the arrangement of chemical elements according to their electron configurations and recurring chemical properties.
learning objectives
- Explain how elements are arranged in the Periodic Table.
A periodic table is a tabular display of the chemical elements, organized on the basis of their atomic numbers, electron configurations, and recurring chemical properties. Elements are presented according to their atomic numbers (number of protons) in increasing order. The standard form of the table comprises an eighteen by seven grid or main body of elements, positioned above a smaller double row of elements. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods. The columns of the s-, d-, and p-blocks are called groups, some of which have names such as the halogens or the noble gases.
Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new elements that are yet to be discovered or synthesized. As a result, a periodic table, in the standard form or some other variant, provides a useful framework for analyzing chemical behavior. Such tables are widely used in chemistry and other sciences.
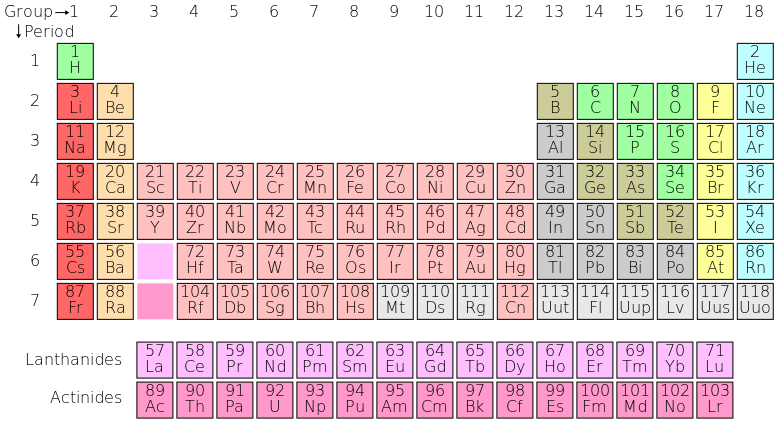
Periodic Table of Elements: The standard form of the periodic table, where the colors represent different categories of elements
The Specifics of the Periodic Table
All versions of the periodic table include only chemical elements, rather than mixtures, compounds, or subatomic particles. Each chemical element has a unique atomic number representing the number of protons in its nucleus. Most elements have differing numbers of neutrons among different atoms: these variants are referred to as isotopes. For example, carbon has three naturally occurring isotopes. All of its atoms have six protons and most have six neutrons as well, but about one percent have seven neutrons, and a very small fraction have eight neutrons. Isotopes are never separated in the periodic table. They are always grouped together under a single element. Elements with no stable isotopes have the atomic masses of their most stable isotopes listed in parentheses.
All elements from atomic numbers ‘1’ (hydrogen) to ‘118’ (ununoctium) have been discovered or synthesized. Of these, elements up through californium exist naturally; the rest have only been synthesized in laboratories. The production of elements beyond ununoctium is being pursued. The question of how the periodic table may need to be modified to accommodate any such additions is a matter of ongoing debate. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.
Although precursors exist, Dmitri Mendeleev is generally credited with the publication of the first widely recognized periodic table in 1869. He developed his table to illustrate periodic trends in the properties of the elements known at the time. Mendeleev also predicted some properties of then-unknown elements that were expected to fill gaps in the table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev’s periodic table has since been expanded and refined with the discovery or synthesis of more new elements and the development of new theoretical models to explain chemical behavior.
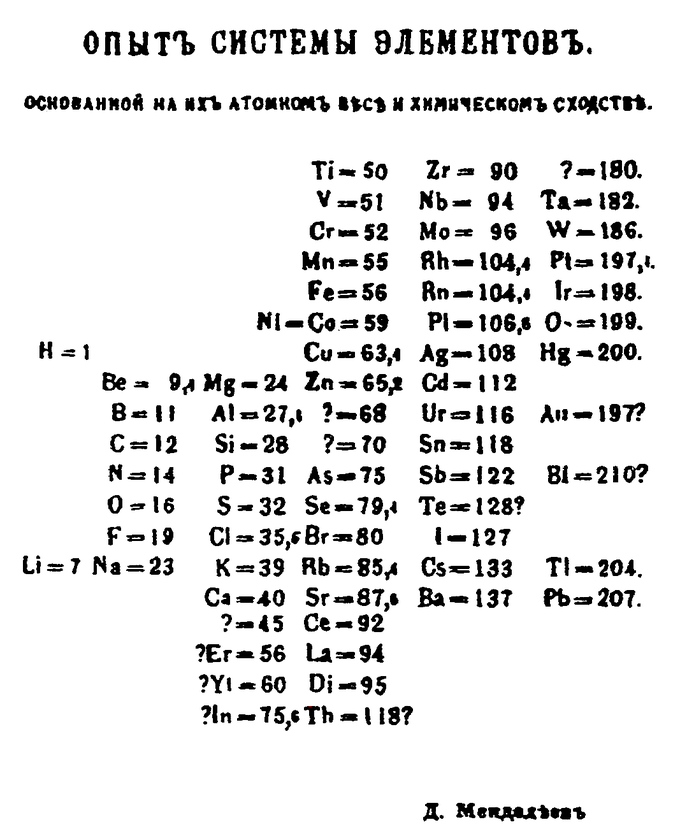
Mendeleev’s 1869 Periodic Table: Mendeleev’s 1869 periodic table presents the periods vertically and the groups horizontally.

Dmitri Mendeleev: Dmitri Mendeleev is known for publishing a widely recognized periodic table.
Electron Configurations
The electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
learning objectives
- Explain the meaning of electron configurations
The electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. Electron configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals.
In atoms, electrons fill atomic orbitals according to the Aufbau principle (shown in ), stated as: a maximum of two electrons are put into orbitals in the order of increasing orbital energy—the lowest-energy orbitals are filled before electrons are placed in higher-energy orbitals. As an example, the electron configuration of the neon atom is 1s2 2s2 2p6 or [He]2s2 2p6, as diagramed in. In molecules, the situation becomes more complex, as each molecule has a different orbital structure. The molecular orbitals are labelled according to their symmetry, rather than the atomic orbital labels used for atoms and monoatomic ions: hence, the electron configuration of the diatomic oxygen molecule, O2, is 1σg2 1σu2 2σg2 2σu2 1πu4 3σg2 1πg2.
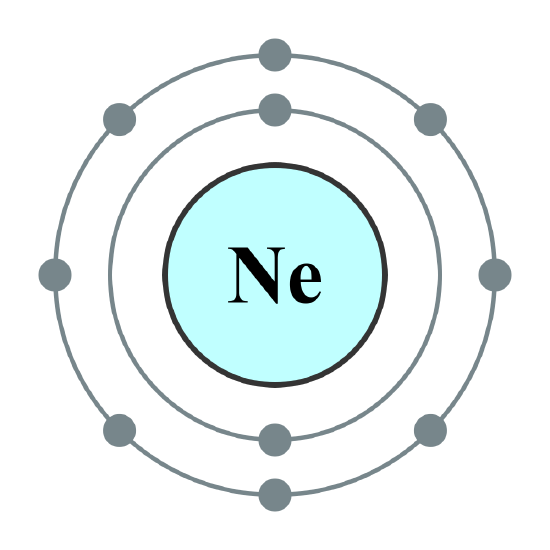
Electron Configuration of Neon Atom: Electron configuration of neon atom showing only outer electron shell.
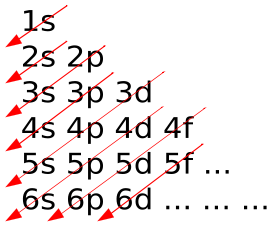
Aufbau Principle: In the Aufbau Principle, as electrons are added to atoms, they are added to the lowest orbitals first.
According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by emission or absorption of a quantum of energy, in the form of a photon.
For atoms or molecules with more than one electron, the motion of electrons are correlated and such picture is no longer exact. An infinite number of electronic configurations are needed to exactly describe any multi-electron system, and no energy can be associated with one single configuration. However, the electronic wave function is usually dominated by a very small number of configurations and therefore the notion of electronic configuration remains essential for multi-electron systems.
Electronic configuration of polyatomic molecules can change without absorption or emission of photon through vibronic couplings.
Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The outermost electron shell is often referred to as the valence shell and (to a first approximation) determines the chemical properties. It should be remembered that the similarities in the chemical properties were remarked more than a century before the idea of electron configuration. The concept of electron configuration is also useful for describing the chemical bonds that hold atoms together. In bulk materials this same idea helps explain the peculiar properties of lasers and semiconductors.
Key Points
- Hydrogen is the only atom in the periodic table that has one electron in the orbitals under ground state.
- In multielectron atoms, the net force on electrons in the outer shells is reduced due to shielding.
- The effective nuclear charge on each electron can be approximated as: Zeff=Z−σZeff=Z−σ, where ZZ is the number of protons in the nucleus and σσ is the average number of electrons between the nucleus and the electron in question.
- A periodic table provides a useful framework for analyzing the chemical behavior of elements.
- A periodic table includes only chemical elements with each chemical element assigned a unique atomic number representing the number of protons in its nucleus.
- Dmitri Mendeleev is credited with the publication of the first widely recognized periodic table in 1869.
- Electrons fill atomic orbitals according to the Aufbau principle in atoms.
- For systems with only one electron, an energy is associated with each electron configuration and electrons are able to move from one configuration to another by emission or absorption of a quantum of energy, in the form of a photon.
- For atoms or molecules with more than one electron, an infinite number of electronic configurations are needed to exactly describe any multi-electron system, and no energy can be associated with one single configuration.
Key Terms
- hydrogen-like: having a single electron
- electron shell: The collective states of all electrons in an atom having the same principal quantum number (visualized as an orbit in which the electrons move).
- valence shell: the outermost shell of electrons in an atom; these electrons take part in bonding with other atoms
- periodic table: A tabular chart of the chemical elements according to their atomic numbers so that elements with similar properties are in the same column.
- element: Any one of the simplest chemical substances that cannot be decomposed in a chemical reaction or by any chemical means and made up of atoms all having the same number of protons.
- atomic number: The number, equal to the number of protons in an atom that determines its chemical properties. Symbol: Z
- atomic orbital: The quantum mechanical behavior of an electron in an atom describing the probability of the electron’s particular position and energy.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- electron shell. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/electron_shell. License: CC BY-SA: Attribution-ShareAlike
- Effective nuclear charge. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Effective_nuclear_charge. License: CC BY-SA: Attribution-ShareAlike
- Electron shielding. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electron_shielding. License: CC BY-SA: Attribution-ShareAlike
- hydrogen-like. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/hydrogen-like. License: CC BY-SA: Attribution-ShareAlike
- valence shell. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/valence_shell. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/b/b3/Effective_Nuclear_Charge.svg/350px-Effective_Nuclear_Charge.svg.png. License: CC BY-SA: Attribution-ShareAlike
- atomic number. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/atomic_number. License: CC BY-SA: Attribution-ShareAlike
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Periodic_table. License: CC BY-SA: Attribution-ShareAlike
- element. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/element. License: CC BY-SA: Attribution-ShareAlike
- periodic table. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/periodic_table. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/b/b3/Effective_Nuclear_Charge.svg/350px-Effective_Nuclear_Charge.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/8/84/Periodic_table.svg/790px-Periodic_table.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/b/b3/Medeleeff_by_repin.jpg/482px-Medeleeff_by_repin.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/b/bb/Mendeleev's_1869_periodic_table.png/487px-Mendeleev's_1869_periodic_table.png. License: CC BY-SA: Attribution-ShareAlike
- Electronic configuration. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electronic_configuration. License: CC BY-SA: Attribution-ShareAlike
- Electron configuration. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Electron_configuration. License: CC BY-SA: Attribution-ShareAlike
- electron shell. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/electron_shell. License: CC BY-SA: Attribution-ShareAlike
- atomic orbital. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/atomic_orbital. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/b/b3/Effective_Nuclear_Charge.svg/350px-Effective_Nuclear_Charge.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/8/84/Periodic_table.svg/790px-Periodic_table.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/b/b3/Medeleeff_by_repin.jpg/482px-Medeleeff_by_repin.jpg. License: CC BY-SA: Attribution-ShareAlike
- Provided by: Wikimedia. Located at: http://upload.wikimedia.org/Wikipedia/commons/thumb/b/bb/Mendeleev's_1869_periodic_table.png/487px-Mendeleev's_1869_periodic_table.png. License: CC BY-SA: Attribution-ShareAlike
- Aufbau principle. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Aufbau_principle. License: CC BY: Attribution
- Provided by: Wikimedia. Located at: upload.wikimedia.org/Wikipedia/commons/thumb/3/3e/Electron_shell_010_Neon_-_no_label.svg/600px-Electron_shell_010_Neon_-_no_label.svg.png. License: CC BY-SA: Attribution-ShareAlike

