8.2: Applications of Quantum Mechanics
- Last updated
- Save as PDF
- Page ID
- 17129
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)learning objectives
- Compare mechanisms of fluorescence and phosphorescence light emission
Fluorescence and Phosphorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of photoluminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. However, when the absorbed electromagnetic radiation is intense, it is possible for one electron to absorb two photons; this two-photon absorption can lead to emission of radiation having a shorter wavelength than the absorbed radiation. The emitted radiation may also be of the same wavelength as the absorbed radiation, termed “resonance fluorescence”.
Fluorescence occurs when an orbital electron of a molecule or atom relaxes to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy. The most striking examples of fluorescence occur when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, and the emitted light is in the visible region.

Fluorescence: Fluorescent minerals emit visible light when exposed to ultraviolet light
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. Excitation of electrons to a higher state is accompanied with the change of a spin state. Once in a different spin state, electrons cannot relax into the ground state quickly because the re-emission involves quantum mechanically forbidden energy state transitions. As these transitions occur very slowly in certain materials, absorbed radiation may be re-emitted at a lower intensity for up to several hours after the original excitation.
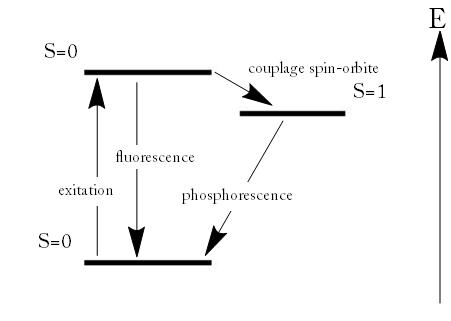
Fluorescence and Phosphorescence: Energy scheme used to explain the difference between fluorescence and phosphorescence
Commonly seen examples of phosphorescent materials are the glow-in-the-dark toys, paint, and clock dials that glow for some time after being charged with a bright light such as in any normal reading or room light. Typically the glowing then slowly fades out within minutes (or up to a few hours) in a dark room.

Phosphorescence: Phosphorescent material glowing in the dark.
Lasers
A laser is a device that emits monochromatic light through a process of optical amplification based on the stimulated emission of photons.
learning objectives
- Identify process that generates laser emission and the defining characteristics of laser light
A laser is a device that emits monochromatic light (electromagnetic radiation ). It does so through a process of optical amplification based on the stimulated emission of photons. The term “laser” originated as an acronym for Light Amplification by Stimulated Emission of Radiation. Laser is distinct from other light sources for its high degree of spatial and temporal coherence, which means that laser outputs a narrow beam that maintains its temporal-phase relationship.
Principles of laser operation are largely based on quantum mechanics. (One exception would be free-electron lasers, whose operation can be explained solely by classical electrodynamics. ) When an electron is excited from a lower-energy to a higher-energy level, it will not stay that way forever. An electron in an excited state may decay to an unoccupied lower-energy state according to a particular time constant characterizing that transition. When such an electron decays without external influence, it emits a photon; this process is called “spontaneous emission. ” The phase associated with the emitted photon is random. A material with many atoms in an excited state may thus result in radiation that is very monochromatic, but the individual photons would have no common phase relationship and would emanate in random directions. This is the mechanism of fluorescence and thermal emission.
However, an external photon at a frequency associated with the atomic transition can affect the quantum mechanical state of the atom. As the incident photon passes by, the rate of transitions of the excited atom can be significantly enhanced beyond that due to spontaneous emission. This “induced” decay process is called stimulated emission. In stimulated emission, the decaying atom produces an identical “copy” of the incoming photon. Therefore, after the atom decays, we have two identical outgoing photons. Since there was only one incoming photon, we amplified the intensity of light by a factor of 2!
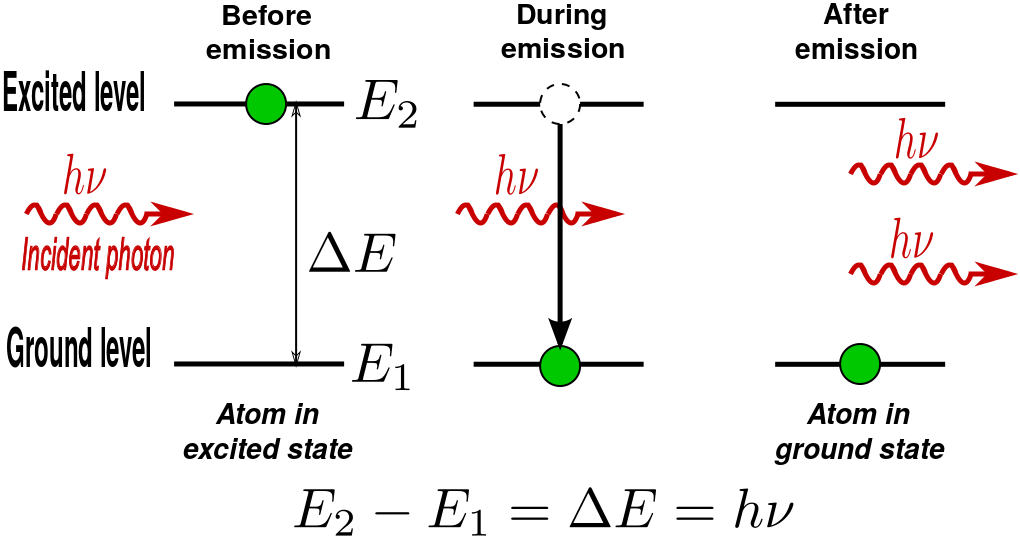
Stimulated Photon Emission: In stimulated emission process, a photon (with a frequency equal to the atomic transition) encounters an excited atom, and a new photon identical to the incoming photon is produced. The result is an atom in the ground state with two outgoing photons.
Holography
Holography is an optical technique which enables three-dimensional images to be made.
learning objectives
- Explain how holographic images are recorded and their properties
Holography is a technique which enables three-dimensional images to be made. It involves the use of a laser, interference, diffraction, light intensity recording and suitable illumination of the recording. The image changes as the position and orientation of the viewing system changes in exactly the same way as if the object were still present, thus making the image appear three-dimensional.
Laser: Holograms are recorded using a flash of light that illuminates a scene and then imprints on a recording medium, much in the way a photograph is recorded. In addition, however, part of the light beam must be shone directly onto the recording medium – this second light beam is known as the reference beam (]). A hologram requires a laser as the sole light source. Laser is required as a light source to produce an interference pattern on the recording plate. To prevent external light from interfering, holograms are usually taken in darkness, or in low level light of a different color from the laser light used in making the hologram. Holography requires a specific exposure time, which can be controlled using a shutter, or by electronically timing the laser
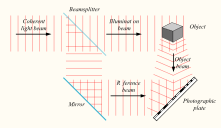
Recording a hologram: Holograms are recorded using a flash of light that illuminates a scene and then imprints on a recording medium, much in the way a photograph is recorded. In addition, however, part of the light beam must be shone directly onto the recording medium – this second light beam is known as the reference beam.
Apparatus: A hologram can be made by shining part of the light beam directly onto the recording medium, and the other part onto the object in such a way that some of the scattered light falls onto the recording medium. A more flexible arrangement for recording a hologram requires the laser beam to be aimed through a series of elements that change it in different ways. The first element is a beam splitter that divides the beam into two identical beams, each aimed in different directions:
- One beam (known as the illumination or object beam) is spread using lenses and directed onto the scene using mirrors. Some of the light scattered (reflected) from the scene then falls onto the recording medium.
- The second beam (known as the reference beam) is also spread through the use of lenses, but is directed so that it doesn’t come in contact with the scene, and instead travels directly onto the recording medium.
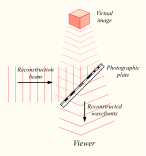
Reconstructing a hologram: An interference pattern can be considered an encoded version of a scene, requiring a particular key – the original light source – in order to view its contents. This missing key is provided later by shining a laser, identical to the one used to record the hologram, onto the developed film. When this beam illuminates the hologram, it is diffracted by the hologram’s surface pattern. This produces a light field identical to the one originally produced by the scene and scattered onto the hologram
Several different materials can be used as the recording medium. One of the most common is a film very similar to photographic film (silver halide photographic emulsion), but with a much higher concentration of light-reactive grains, making it capable of the much higher resolution that holograms require. A layer of this recording medium (e.g. silver halide) is attached to a transparent substrate, which is commonly glass, but may also be plastic.
Process: When the two laser beams reach the recording medium, their light waves intersect and interfere with each other. It is this interference pattern that is imprinted on the recording medium. The pattern itself is seemingly random, as it represents the way in which the scene’s light interfered with the original light source – but not the original light source itself. The interference pattern can be considered an encoded version of the scene, requiring a particular key – the original light source – in order to view its contents.
This missing key is provided later by shining a laser, identical to the one used to record the hologram, onto the developed film. When this beam illuminates the hologram, it is diffracted by the hologram’s surface pattern. This produces a light field identical to the one originally produced by the scene and scattered onto the hologram. The image this effect produces in a person’s retina is known as a virtual image.
The Periodic Table of Elements
A periodic table is a tabular display of elements organized by their atomic numbers, electron configurations, and chemical properties.
learning objectives
- Explain how properties of elements vary within groups and across periods in the periodic table
The periodic table is a tabular display of the chemical elements. The elements are organized based on their atomic numbers, electron configurations, and recurring chemical properties.
In the periodic table, elements are presented in order of increasing atomic number (the number of protons). The rows of the table are called periods; the columns of the s- (columns 1-2 and He), d- (columns 3-12), and p-blocks (columns 13-18, except He) are called groups. (The terminology of s-, p-, and d- blocks originate from the valence atomic orbitals the element’s electrons occupy. ) Some groups have specific names, such as the halogens or the noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet-to-be-discovered, or synthesized elements. As a result, the periodic table provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences.
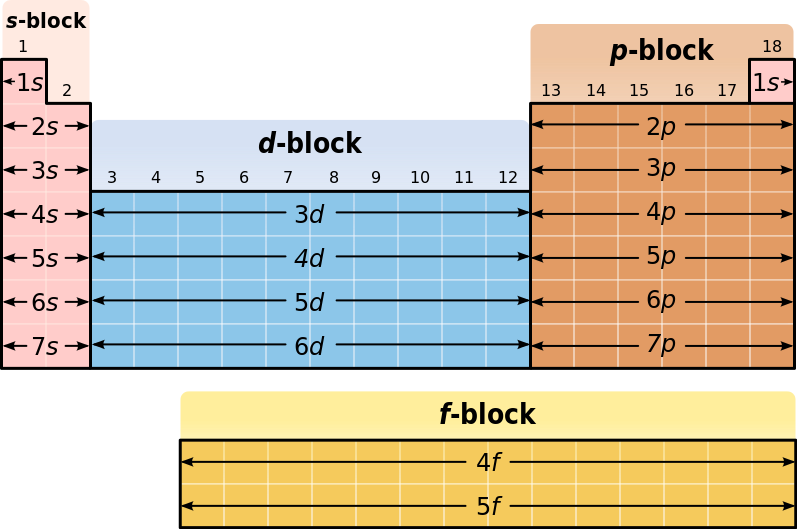
Blocks in the Periodic Table: A diagram of the periodic table, highlighting the different blocks
History of the Periodic Table
Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. Mendeleev designed the table in such a way that recurring (“periodic”) trends in the properties of the elements could be shown. Using the trends he observed, he even left gaps for those elements that he thought were “missing. ” He even predicted the properties that he thought the missing elements would have when they were discovered. Many of these elements were indeed later discovered, and Mendeleev’s predictions were proved to be correct.
Groups
Agroup, or family, is a vertical column in the periodic table. Groups usually have more significant periodic trends than do periods and blocks, which are explained below. Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements in the same group generally have the same electron configurations in their valence (or outermost, partially filled) shell. Consequently, elements in the same group tend to have shared chemistry and exhibit a clear trend in properties with increasing atomic number. However, in some parts of the periodic table, such as the d-block and the f-block, horizontal similarities can be as important as, or more pronounced than, vertical similarities.
Periods
A period is a horizontal row in the periodic table. Although groups generally have more significant periodic trends, there are regions where horizontal trends are more significant than vertical group trends, such as in the f-block, where the lanthanides and actinides form two substantial horizontal series of elements. Elements in the same period show trends in atomic radius, ionization energy, and electron affinity. Atomic radius usually decreases from left to right across a period. This occurs because each successive element has an added proton and electron, which causes the electron to be drawn closer to the nucleus, decreasing the radius.
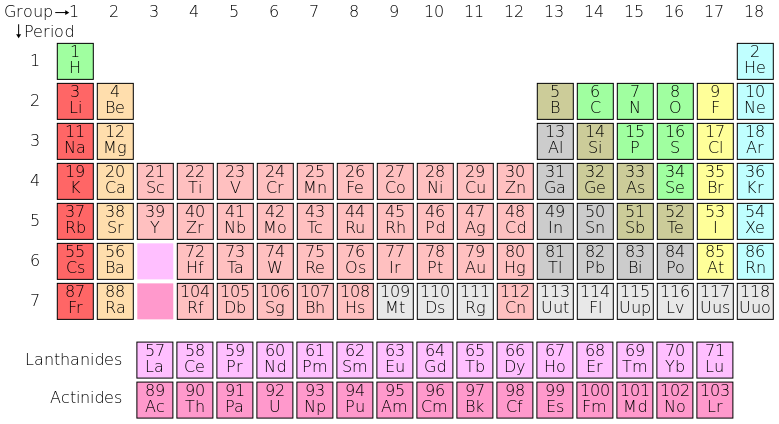
The periodic table: Here is the complete periodic table with atomic numbers, groups, and periods. Each entry on the periodic table represents one element, and compounds are made up of several of these elements.
X-Rays
X-rays are a form of electromagnetic radiation and have wavelengths in the range of 0.01 to 10 nanometers.
learning objectives
- Describe the properties of X-rays and how can be generated
X-radiation (composed of x-rays) is a form of electromagnetic radiation. X-rays have wavelengths in the range of 0.01 to 10 nanometers, which corresponds to frequencies in the range of 30 petahertz to 30 exahertz (3·1016 Hz to 3·1019 Hz) and energies in the of range 100 eV to 100 keV.
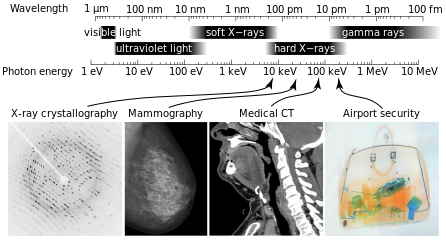
X-Ray Spectrum and Applications: X-rays are part of the electromagnetic spectrum, with wavelengths shorter than those of visible light. Different applications use different parts of the X-ray spectrum.
X-rays can be generated by an x-ray tube, a vacuum tube that uses high voltage to accelerate the electrons released by a hot cathode to a high velocity. The high-velocity electrons collide with a metal target, the anode, creating the x-rays. The maximum energy of the produced x-ray photon is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so an 80-kV tube cannot create x-rays with an energy greater than 80 keV. When the electrons hit the target, x-rays are created through two different atomic processes:
- X-ray fluorescence, if the electron has enough energy that it can knock an orbital electron out of the inner electron shell of a metal atom. As a result, electrons from higher energy levels fill up the vacancy, and x-ray photons are emitted. This process produces an emission spectrum of x-rays at a few discrete frequencies, sometimes referred to as the spectral lines. The spectral lines generated depend on the target (anode) element used and therefore are called characteristic lines. Usually these are transitions from upper shells into the K shell (called K lines), or the L shell (called L lines), and so on.
- Bremsstrahlung, literally meaning braking radiation. Bremsstrahlung is radiation given off by the electrons as they are scattered by the strong electric field near the high-Z (proton number) nuclei. These x-rays have a continuous spectrum. The intensity of the x-rays increases linearly with decreasing frequency, from zero at the energy of the incident electrons, the voltage on the x-ray tube.
Both of these x-ray production processes are inefficient, with a production efficiency of only about one percent. Therefore, to produce a usable flux of x-rays, most of the electric power consumed by the tube is released as heat waste. The x-ray tube must be designed to dissipate this excess heat.
A specialized source of x-rays that is becoming widely used in research is synchrotron radiation, which is generated by particle accelerators. Its unique features are x-ray outputs many orders of magnitude greater than those of x-ray tubes, wide x-ray spectra, excellent collimation, and linear polarization.
Quantum-Mechanical View of Atoms
Atom is a basic unit of matter that consists of a nucleus surrounded by negatively charged electron cloud, commonly called atomic orbitals.
learning objectives
- Identify major contributions to the understanding of atomic structure that were made by Niels Bohr, Erwin Schrödinger, and Werner Heisenberg
The atom is a basic unit of matter that consists of a nucleus surrounded by negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons. The electrons of an atom are bound to the nucleus by the electromagnetic (Coulomb) force. Atoms are minuscule objects with diameters of a few tenths of a nanometer and tiny masses proportional to the volume implied by these dimensions. Atoms in solid states (or, to be precise, their electron clouds) can be observed individually using special instruments such as the scanning tunneling microscope.
Hydrogen-1 (one proton + one electron) is the simplest form of atoms, and not surprisingly, our quantum mechanical understanding of atoms evolved with the understanding of this species. In 1913, physicist Niels Bohr suggested that the electrons were confined into clearly defined, quantized orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states. An electron must absorb or emit specific amounts of energy to transition between these fixed orbits. Bohr’s model successfully explained spectroscopic data of hydrogen very well, but it adopted a semiclassical approach where electron was still considered a (classical) particle.
Adopting Louis de Broglie’s proposal of wave-particle duality, Erwin Schrödinger, in 1926, developed a mathematical model of the atom that described the electrons as three-dimensional waveforms rather than point particles. A consequence of using waveforms to describe particles is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at the same time; this became known as the uncertainty principle, formulated by Werner Heisenberg in 1926. Thereafter, the planetary model of the atom was discarded in favor of one that described atomic orbital zones around the nucleus where a given electron is most likely to be observed.
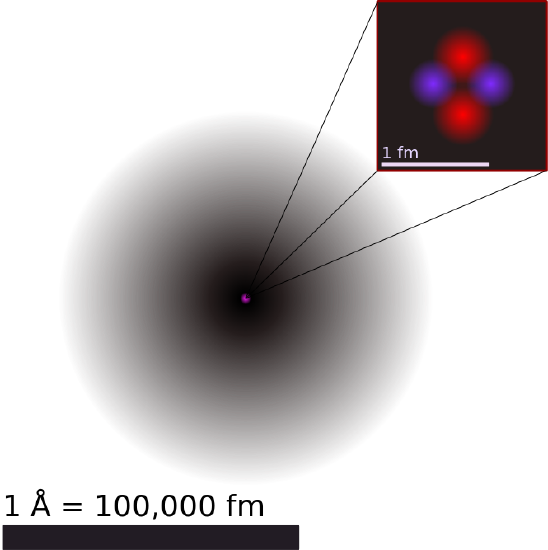
Illustration of the Helium Atom: This is an illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10-10 m, or 100 pm).
Modern quantum mechanical view of hydrogen has evolved further after Schrödinger, by taking relativistic correction terms into account. Quantum electrodynamics (QED), a relativistic quantum field theory describing the interaction of electrically charged particles, has successfully predicted minuscule corrections in energy levels. One of the hydrogen’s atomic transitions (n=2 to n=1, n: principal quantum number) has been measured to an extraordinary precision of 1 part in a hundred trillion. This kind of spectroscopic precision allows physicists to refine quantum theories of atoms, by accounting for minuscule discrepancies between experimental results and theories.
Key Points
- The emitted light usually has a longer wavelength, and therefore lower energy, than the absorbed radiation.
- Fluorescence occurs when an orbital electron of a molecule or atom relaxes to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy.
- In a phosphorescence, excitation of electrons to a higher state is accompanied with the change of a spin state. Relaxation is a slow process since it involves energy state transitions “forbidden” in quantum mechanics.
- Principles of laser operation are largely based on quantum mechanics, most importantly on the process of the stimulated emission of photons.
- Spontaneous emission is a random decaying process. The phase associated with the emitted photon is also random.
- Atomic transition can be stimulated by the presence of an incoming photon at a frequency associated with the atomic transition. This process leads to optical amplification as an identical photon is emitted along with the incoming photon.
- When the two laser beams reach the recording medium, their light waves intersect and interfere with each other. It is this interference pattern that is imprinted on the recording medium.
- When a reconstruction beam illuminates the hologram, it is diffracted by the hologram’s surface pattern. This produces a light field identical to the one originally produced by the scene and scattered onto the hologram.
- Holographic image changes as the position and orientation of the viewing system changes in exactly the same way as if the object were still present, thus making the image appear three-dimensional.
- A periodic table is a useful framework for analyzing chemical behavior. Such tables are widely used in chemistry and other sciences.
- A group, or family, is a vertical column in the periodic table. Groups usually have more significant periodic trends than do periods and blocks.
- A period is a horizontal row in the periodic table. Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity.
- X-rays can be generated by an x-ray tube, a vacuum tube, or a particle accelerator.
- X-ray fluorescence and Bremsstrahlung are processes through which x-rays are produced.
- Synchrotron radiation is generated by particle accelerators. Its unique features are x-ray outputs many orders of magnitude greater than those of x-ray tubes, wide x-ray spectra, excellent collimation, and linear polarization.
- Niels Bohr suggested that the electrons were confined into clearly defined, quantized orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states.
- Erwin Schrödinger, in 1926, developed a mathematical model of the atom that described the electrons as three-dimensional waveforms rather than point particles.
- Modern quantum mechanical view of hydrogen has evolved further after Schrödinger, by taking relativistic correction terms into account. This is referred to a quantum electrodynamics (QED).
Key Terms
- spin: A quantum angular momentum associated with subatomic particles; it also creates a magnetic moment.
- photon: The quantum of light and other electromagnetic energy, regarded as a discrete particle having zero rest mass, no electric charge, and an indefinitely long lifetime.
- ground state: the stationary state of lowest energy of a particle or system of particles
- free-electron laser: a laser that use a relativistic electron beam as the lasing medium, which moves freely through a magnetic structure
- monochromatic: Describes a beam of light with a single wavelength (i.e., of one specific color or frequency).
- coherence: an ideal property of waves that enables stationary (i.e., temporally and spatially constant) interference
- interference: An effect caused by the superposition of two systems of waves, such as a distortion on a broadcast signal due to atmospheric or other effects.
- laser: A device that produces a monochromatic, coherent beam of light.
- silver halide: The light-sensitive chemicals used in photographic film and pape
- atomic orbital: The quantum mechanical behavior of an electron in an atom describing the probability of the electron’s particular position and energy.
- electron affinity: the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion
- ionization energy: the amount of energy required to remove an electron from an atom or molecule in the gas phase
- photon: The quantum of light and other electromagnetic energy, regarded as a discrete particle having zero rest mass, no electric charge, and an indefinitely long lifetime.
- particle accelerator: A device that accelerates electrically charged particles to extremely high speeds, for the purpose of inducing high-energy reactions or producing high-energy radiation.
- wave-particle duality: A postulation that all particles exhibit both wave and particle properties. It is a central concept of quantum mechanics.
- scanning tunneling microscope: An instrument for imaging surfaces at the atomic level.
- semiclassical approach: A theory in which one part of a system is described quantum-mechanically whereas the other is treated classically.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- ground state. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ground_state. License: CC BY-SA: Attribution-ShareAlike
- Fluorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Fluorescence%23Physical_principles. License: CC BY-SA: Attribution-ShareAlike
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY-SA: Attribution-ShareAlike
- photon. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/photon. License: CC BY-SA: Attribution-ShareAlike
- spin. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/spin. License: CC BY-SA: Attribution-ShareAlike
- Fluorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Fluorescence%23Physical_principles. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Laser. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Laser%23Types_and_operating_principles. License: CC BY-SA: Attribution-ShareAlike
- Laser. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Laser%23Gain_medium_and_cavity. License: CC BY-SA: Attribution-ShareAlike
- coherence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/coherence. License: CC BY-SA: Attribution-ShareAlike
- free-electron laser. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/free-electron%20laser. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/definition/monochromatic. License: CC BY-SA: Attribution-ShareAlike
- Fluorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Fluorescence%23Physical_principles. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Stimulated emission. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Stimulated_emission. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY-SA: Attribution-ShareAlike
- laser. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/laser. License: CC BY-SA: Attribution-ShareAlike
- interference. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/interference. License: CC BY-SA: Attribution-ShareAlike
- silver halide. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/silver%20halide. License: CC BY-SA: Attribution-ShareAlike
- Fluorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Fluorescence%23Physical_principles. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Stimulated emission. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Stimulated_emission. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Free High School Science Texts Project, The Periodic Table: Groups and Periods. September 17, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m38760/latest/. License: CC BY: Attribution
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Periodic_table. License: CC BY-SA: Attribution-ShareAlike
- electron affinity. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/electron%20affinity. License: CC BY-SA: Attribution-ShareAlike
- ionization energy. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ionization_energy. License: CC BY-SA: Attribution-ShareAlike
- atomic orbital. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/atomic_orbital. License: CC BY-SA: Attribution-ShareAlike
- Fluorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Fluorescence%23Physical_principles. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Stimulated emission. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Stimulated_emission. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/File:Periodic_table.svg. License: CC BY: Attribution
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Periodic_table. License: CC BY: Attribution
- X-ray. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/X-ray. License: CC BY-SA: Attribution-ShareAlike
- photon. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/photon. License: CC BY-SA: Attribution-ShareAlike
- particle accelerator. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/particle_accelerator. License: CC BY-SA: Attribution-ShareAlike
- Fluorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Fluorescence%23Physical_principles. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Stimulated emission. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Stimulated_emission. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/File:Periodic_table.svg. License: CC BY: Attribution
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Periodic_table. License: CC BY: Attribution
- X-rays. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/X-rays. License: Public Domain: No Known Copyright
- Atom. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Atom. License: CC BY-SA: Attribution-ShareAlike
- Quantum electrodynamics. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Quantum_electrodynamics. License: CC BY-SA: Attribution-ShareAlike
- Theodor W. Hu00e4nsch. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Theodor_W._H%C3%A4nsch. License: CC BY-SA: Attribution-ShareAlike
- scanning tunneling microscope. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/scanning%20tunneling%20microscope. License: CC BY-SA: Attribution-ShareAlike
- wave-particle duality. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/wave-particle%20duality. License: CC BY-SA: Attribution-ShareAlike
- semiclassical approach. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/semiclassical%20approach. License: CC BY-SA: Attribution-ShareAlike
- Fluorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Fluorescence%23Physical_principles. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Phosphorescence. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Phosphorescence. License: CC BY: Attribution
- Stimulated emission. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Stimulated_emission. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Holography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Holography. License: CC BY: Attribution
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/File:Periodic_table.svg. License: CC BY: Attribution
- Periodic table. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Periodic_table. License: CC BY: Attribution
- X-rays. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/X-rays. License: Public Domain: No Known Copyright
- Atom. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Atom. License: CC BY: Attribution

