21.1: Molecules — an Analogy
- Page ID
- 32881
Molecules are bound states of two or more atoms. In chemistry we identify several modes of molecular binding, e. g., covalent and ionic bonds, the hydrogen bond, and binding at low temperatures due to the van der Waal’s force. All of these bonds involve electromagnetic forces, but all (except arguably the ionic bond) are relatively subtle residual forces between atoms that are electrically neutral. The ways in which atoms form molecules are therefore complex and resistent to accurate calculation.
Atomic nuclei are the nuclear equivalent of molecules, in that they are bound states of nucleons, which are themselves “uncharged” composite particles. The charge we refer to here is not the electric charge (nuclei do of course possess this!), but the strong or color charge. As we discovered in the previous chapter, nucleons are color-neutral combinations of quarks. Thus, the “strong” forces between nucleons are subtle residuals of inter-quark forces. This is reflected in the binding energies; quark-quark binding energies are on the order of the rest energies of the quarks themselves. However, nuclear binding energies are typically of order 10 MeV per nucleon, or about 1% of the rest energy of a nucleon.
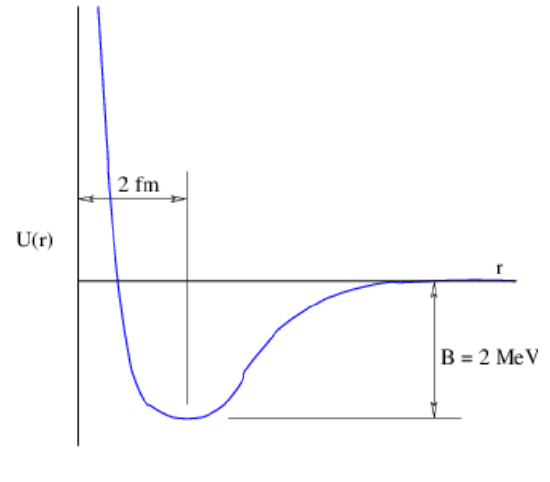
The residual nature of nuclear forces makes them complex and difficult to calculate from our basic knowledge of quantum chromodynamics for the same reasons that intermolecular forces are difficult to calculate. An empirical approach is thus needed in order to understand their effects.
In contrast to molecules and atomic nuclei, atoms are relatively easy to understand. This is true for two reasons: (1) Electrons appear to be truly fundamental point particles. (2) Though the atomic nucleus itself is a very complex system, little of this complexity spills over into atomic calculations, because on the atomic scale the nucleus is very nearly a point particle. Thus, both main ingredients in atoms are “simple” from the point of view of atomic calculations.
The above result is true because by some accident of nature, the mass of the electron is so much less than the masses of quarks. It would be interesting to speculate what atomic theory would be like if this weren’t true — there would be no scale separation between the atomic and nuclear scales, and the world would be a very different place!


