11.3: Radioactive Decay
- Page ID
- 76709
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)By the end of this section, you will be able to:
- Describe the decay of a radioactive substance in terms of its decay constant and half-life
- Use the radioactive decay law to estimate the age of a substance
- Explain the natural processes that allow the dating of living tissue using 14C
In 1896, Antoine Becquerel discovered that a uranium-rich rock emits invisible rays that can darken a photographic plate in an enclosed container. Scientists offer three arguments for the nuclear origin of these rays. First, the effects of the radiation do not vary with chemical state; that is, whether the emitting material is in the form of an element or compound. Second, the radiation does not vary with changes in temperature or pressure—both factors that in sufficient degree can affect electrons in an atom. Third, the very large energy of the invisible rays (up to hundreds of eV) is not consistent with atomic electron transitions (only a few eV). Today, this radiation is explained by the conversion of mass into energy deep within the nucleus of an atom. The spontaneous emission of radiation from nuclei is called nuclear radioactivity (Figure \(\PageIndex{1}\)).

Radioactive Decay Law
When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. Radioactive decay occurs for all nuclei with \(Z > 82\), and also for some unstable isotopes with \(Z < 83\). The decay rate is proportional to the number of original (undecayed) nuclei N in a substance. The number of nuclei lost to decay, \(-dN\) in time interval dt, is written
\[-\dfrac{dN}{dt} = \lambda N \label{eq2} \]
where \(\lambda\) is called the decay constant. (The minus sign indicates the number of original nuclei decreases over time.) In other words, the more nuclei available to decay, the more that do decay (in time dt). Equation \ref{eq2} can be rewritten as
\[\dfrac{dN}{N} = -\lambda dt. \nonumber \]
Integrating both sides of the equation, and defining \(N_0\) to be the number of nuclei at \(t = 0\), we obtain
\[\int_{N_0}^N \dfrac{dN'}{N} = - \int_0^t \lambda dt'. \nonumber \]
This gives us
\[\ln\dfrac{N}{N_0} = -\lambda t. \label{eq4} \]
Taking the left and right sides of Equation \ref{eq4} as a power of \(e\), we have the radioactive decay law.
The total number \(N\) of radioactive nuclei remaining after time \(t\) is
\[N = N_0e^{-\lambda t} \label{decay law} \]
where \(\lambda\) is the decay constant for the particular nucleus.
The total number of nuclei drops very rapidly at first, and then more slowly (Figure \(\PageIndex{2}\)).
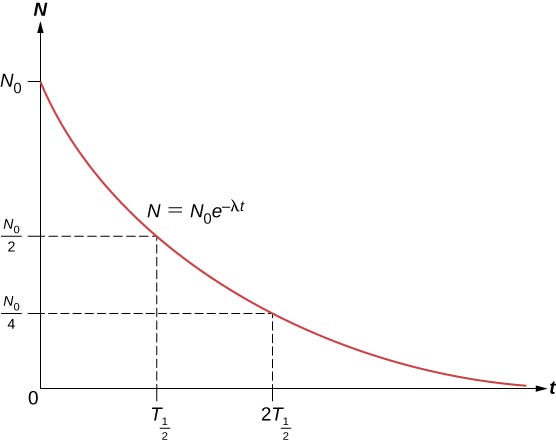
The half-life \((T_{1/2})\) of a radioactive substance is defined as the time for half of the original nuclei to decay (or the time at which half of the original nuclei remain). The half-lives of unstable isotopes are shown in the chart of nuclides. The number of radioactive nuclei remaining after an integer (n) number of half-lives is therefore
\[N = \dfrac{N_0}{2^n} \label{eq5} \]
If the decay constant \((\lambda)\) is large, the half-life is small, and vice versa. To determine the relationship between these quantities, note that when \(t = T_{1/2}\), then \(N = N_0/2\).
Thus, Equation \ref{eq5} can be rewritten as
\[\dfrac{N_0}{2} = N_0e^{-\lambda T_{1/2}}. \nonumber \]
Dividing both sides by \(N_0\) and taking the natural logarithm yields
\[\ln \dfrac{1}{2} = \ln \, e^{-\lambda T_{1/2}} \nonumber \]
which reduces to
\[\lambda = \dfrac{0.693}{T_{1/2}}. \nonumber \]
Thus, if we know the half-life T1/2 of a radioactive substance, we can find its decay constant. The lifetime \(\overline{T}\) of a radioactive substance is defined as the average amount of time that a nucleus exists before decaying. The lifetime of a substance is just the reciprocal of the decay constant, written as
\[\overline{T} = \dfrac{1}{\lambda}. \nonumber \]
The activity A is defined as the magnitude of the decay rate, or
\[A = -\dfrac{dN}{dt} = \lambda N = \lambda N_0 e^{-\lambda t}. \nonumber \]
The infinitesimal change dN in the time interval dt is negative because the number of parent (undecayed) particles is decreasing, so the activity (A) is positive. Defining the initial activity as \(A_0 = \lambda N_0\), we have
\[A = A_0 e^{-\lambda t}. \label{eq8} \]
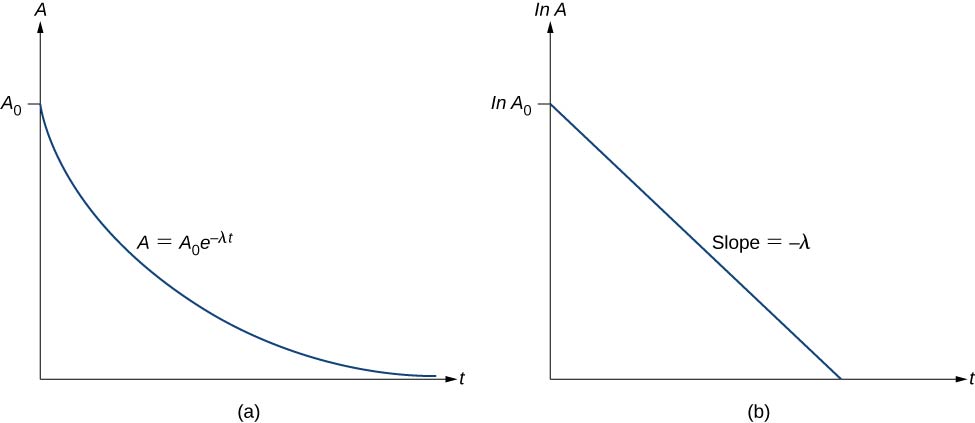
Thus, the activity A of a radioactive substance decreases exponentially with time (Figure \(\PageIndex{3}\)).
The half-life of strontium-90, \(\ce{_{38}^{90}Sr}\), is 28.8 y. Find (a) its decay constant and (b) the initial activity of 1.00 g of the material.
Strategy
We can find the decay constant directly from Equation \ref{eq8}. To determine the activity, we first need to find the number of nuclei present.
Solution
a. The decay constant is found to be
\[\lambda = \dfrac{0.693}{T_{1/2}} = \left(\dfrac{0.693}{T_{1/2}}\right)\left(\dfrac{1 \, yr}{3.16 \times 10^7 \, s}\right) = 7.61 \times 10^{-10} \, s^{-1}. \nonumber \]
b. The atomic mass of \(_{38}^{90}Sr\) is 89.91 g. Using Avogadro’s number \(N_A = 6.022 \times 10^{23}\) atoms/mol, we find the initial number of nuclei in 1.00 g of the material:
\[N_0 = \dfrac{1.00 \, g}{89.91 \, g} (N_A) = 6.70 \times 10^{21} \, nuclei. \nonumber \]
From this, we find that the activity \(A_0\) at \(t = 0\) for 1.00 g of strontium-90 is
\[A_0 = \lambda N_0 = (7.61 \times 10^{-10} s^{-1})(6.70 \times 10^{21} \, nuclei) = 5.10 \times 10^{12} \, decays/s. \nonumber \]
Expressing \(\lambda\) in terms of the half-life of the substance, we get
\[A = A_0 e^{-(0.693/T_{1/2})T_{1/2}} = A_0 e^{-0.693} = A_0/2. \label{eq11} \]
Therefore, the activity is halved after one half-life. We can determine the decay constant \(\lambda\) by measuring the activity as a function of time. Taking the natural logarithm of the left and right sides of Equation \ref{eq11}, we get
\[\ln \, A = - \lambda t + \ln \, A_0. \nonumber \]
This equation follows the linear form \(y = mx + b\). If we plot \ln A versus t, we expect a straight line with slope \(-\lambda\) and y-intercept \(\ln \, A_0\) (Figure \(\PageIndex{3b}\)). Activity A is expressed in units of becquerels (Bq), where one \(1 \, Bq = 1 \, decay \, per \, second\). This quantity can also be expressed in decays per minute or decays per year. One of the most common units for activity is the curie (Ci), defined to be the activity of 1 g of \(^{226}Ra\). The relationship between the Bq and Ci is
\[1 \, Ci = 3.70 \times 10^{10}Bq. \nonumber \]
Approximately \(20\%\) of the human body by mass is carbon. Calculate the activity due to \(^{14}C\) in 1.00 kg of carbon found in a living organism. Express the activity in units of Bq and Ci.
Strategy
The activity of \(^{14}C\) is determined using the equation \(A_0 = \lambda N_0\), where λ is the decay constant and \(N_0\) is the number of radioactive nuclei. The number of \(^{14}C\) nuclei in a 1.00-kg sample is determined in two steps. First, we determine the number of \(^{12}C\) nuclei using the concept of a mole. Second, we multiply this value by \(1.3 \times 10^{-12}\) (the known abundance of \(^{14}C\) in a carbon sample from a living organism) to determine the number of \(^{14}C\) nuclei in a living organism. The decay constant is determined from the known half-life of \(^{14}C\) (available from [link]).
Solution
One mole of carbon has a mass of 12.0 g, since it is nearly pure \(^{12}C\). Thus, the number of carbon nuclei in a kilogram is
\[N(^{12}C) = \dfrac{6.02 \times 10^{23} mol^{-1}}{12.0 \, g/mol} \times (1000 \, g) = 5.02 \times 10^{25}. \nonumber \]
The number of \(^{14}C\) nuclei in 1 kg of carbon is therefore
\[N(^{14}C) = (5.02 \times 10^{25})(1.3 \times 10^{_12}) = 6.52 \times 10^{13}. \nonumber \]
Now we can find the activity \(A\) by using Equation \ref{eq11}. Entering known values gives us
\[A = \dfrac{0.693 (6.52 \times 10^{13})}{5730 \, y} = 7.89 \times 10^9 \, y^{-1} \nonumber \]
or \(7.89 \times 10^9\) decays per year. To convert this to the unit Bq, we simply convert years to seconds. Thus,
\[A = (7.89 \times 10^9 \, y^{-1}) \dfrac{1.00 \, y}{3.16 \times 10^7 \, s} = 250 \, Bq, \nonumber \]
or 250 decays per second. To express A in curies, we use the definition of a curie,
\[A = \dfrac{250 \, Bq}{3.7 \times 10^{10} \, Bq/Ci} = 6.76 \times 10^{-9} Ci. \nonumber \]
Thus,
\[A = 6.76 \, nCi. \nonumber \]
Significance
Approximately \(20\%\) of the human body by weight is carbon. Hundreds of \(^{14}C\) decays take place in the human body every second. Carbon-14 and other naturally occurring radioactive substances in the body compose a person’s background exposure to nuclear radiation. As we will see later in this chapter, this activity level is well below the maximum recommended dosages.
Radioactive Dating
Radioactive dating is a technique that uses naturally occurring radioactivity to determine the age of a material, such as a rock or an ancient artifact. The basic approach is to estimate the original number of nuclei in a material and the present number of nuclei in the material (after decay), and then use the known value of the decay constant \(\lambda\) and Equation \ref{decay law}to calculate the total time of the decay, \(t\).
An important method of radioactive dating is carbon-14 dating. Carbon-14 nuclei are produced when high-energy solar radiation strikes \(^{14}N\) nuclei in the upper atmosphere and subsequently decay with a half-life of 5730 years. Radioactive carbon has the same chemistry as stable carbon, so it combines with the ecosphere and eventually becomes part of every living organism. Carbon-14 has an abundance of 1.3 parts per trillion of normal carbon. Therefore, if you know the number of carbon nuclei in an object, you multiply that number by \(1.3 \times 10^{-12}\) to find the number of \(^{14}C\) nuclei in that object. When an organism dies, carbon exchange with the environment ceases, and \(^{14}C\) is not replenished as it decays.
By comparing the abundance of \(^{14}C\) in an artifact, such as mummy wrappings, with the normal abundance in living tissue, it is possible to determine the mummy’s age (or the time since the person’s death). Carbon-14 dating can be used for biological tissues as old as 50,000 years, but is generally most accurate for younger samples, since the abundance of \(^{14}C\) nuclei in them is greater. Very old biological materials contain no \(^{14}C\) at all. The validity of carbon dating can be checked by other means, such as by historical knowledge or by tree-ring counting.
In an ancient burial cave, your team of archaeologists discovers ancient wood furniture. Only \(80\%\) of the original \(^{14}C\) remains in the wood. How old is the furniture?
Strategy
The problem statement implies that \(N/N_0 = 0.80\). Therefore, we rearrange Equation \ref{decay law} to find the product, \(\lambda t\). We know the half-life of \(^{14}C\) is 5730 y, so we also know the decay constant, and therefore the total decay time \(t\).
Solution
We rearrange Equation \ref{decay law} for \(N/N_0\) to gives
\[\dfrac{N}{N_0} = e^{-\lambda t}. \nonumber \]
Thus
\[0.80 = e^{-\lambda t}. \nonumber \]
Taking the natural logarithm of both sides yields
\[\ln \, 0.80 = - \lambda t,\nonumber \]
so that
\[-0.223 = -\lambda t. \nonumber \]
Rearranging the equation to isolate \(t\) gives us
\[t = \dfrac{0.223}{\left(\dfrac{0.693}{5730 \, y}\right)} = 1844 \, y. \nonumber \]
Significance
The furniture is almost 2000 years old—an impressive discovery. The typical uncertainty on carbon-14 dating is about \(5\%\), so the furniture is anywhere between 1750 and 1950 years old. This date range must be confirmed by other evidence, such as historical records.
A radioactive nuclide has a high decay rate. What does this mean for its half-life and activity?
- Answer
-
Half-life is inversely related to decay rate, so the half-life is short. Activity depends on both the number of decaying particles and the decay rate, so the activity can be great or small.
Visit the Radioactive Dating Game to learn about the types of radiometric dating and try your hand at dating some ancient objects.


