14.22: Introduction
- Last updated
- Jun 17, 2019
- Save as PDF
- Page ID
- 18335

- Boundless
- Boundless
( \newcommand{\kernel}{\mathrm{null}\,}\)
learning objectives
- Analyze the necessity to exclude energy transferred between system as heat from mechanical work
Work
In thermodynamics, work performed by a closed system is the energy transferred to another system that is measured by mechanical constraints on the system. Thermodynamic work encompasses mechanical work (gas expansion, ) plus many other types of work, such as electrical. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. It necessarily excludes energy transferred between systems as heat, which is modeled distinctly in thermodynamics. For closed systems, energy changes in a system other than as work transfer are as heat.
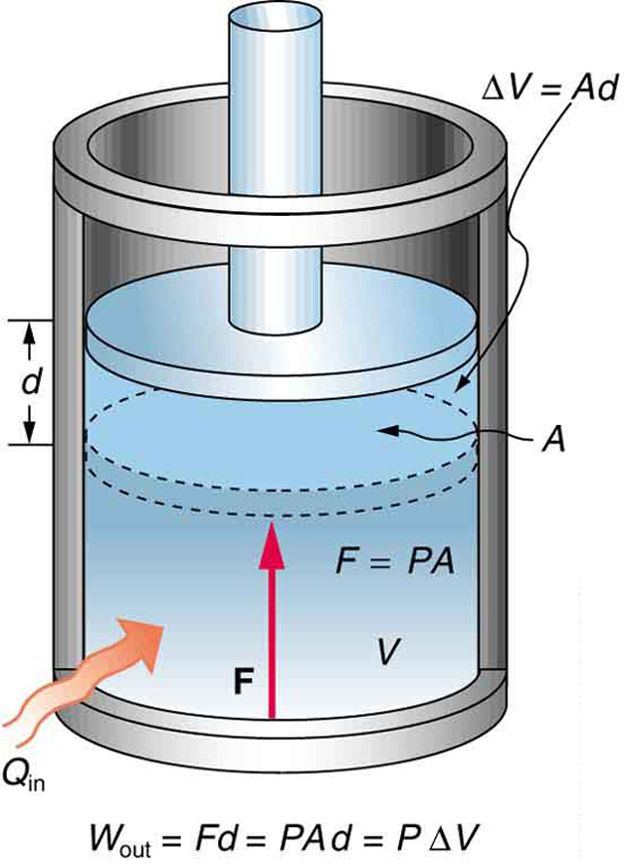
Fig 1: An isobaric expansion of a gas requires heat transfer during the expansion to keep the pressure constant. Since pressure is constant, the work done is PΔV.
Heat and Work
Heat transfer (often represented by Q) and doing work (W) are the two everyday means of bringing energy into or taking energy out of a system. The processes are quite different. Heat transfer, a less organized process, is driven by temperature differences. Work, a quite organized process(as in gas expansion), involves a macroscopic force exerted through a distance. Nevertheless, heat and work can produce identical results. Both heat and work can cause a temperature increase.
Heat transfer into a system, such as when the Sun warms the air in a bicycle tire, can increase its temperature, and so can work done on the system, as when the bicyclist pumps air into the tire. Once the temperature increase has occurred, it is impossible to tell whether it was caused by heat transfer or by doing work.
This uncertainty is an important point. Heat transfer and work are both energy in transit—neither is stored as such in a system. However, both can change the internal energy of a system. Internal energy is a form of energy completely different from either heat or work.
A Review of the Zeroth Law
Zeroth law justifies the use of thermodynamic temperature, defined as the shared temperature of three designated systems at equilibrium.
learning objectives
- Discuss how the Zeroth Law of Thermodynamics justifies the use of thermodynamic temperature
Thermodynamics and PV Diagrams: A brief introduction to the zeroth and 1st laws of thermodynamics as well as PV diagrams for students.

Thermometer: A thermometer calibrated in degrees Celsius
The Zeroth Law of Thermodynamics states: If two systems, A and B, are in thermal equilibrium with each other, and B is in thermal equilibrium with a third system, C, then A is also in thermal equilibrium with C.
This law was postulated in the 1930s, after the first and second laws of thermodynamics had been developed and named. It is called the “zeroth” law because it comes logically before the first and second laws (discussed in Atoms on the 1st and 2nd laws).
Two systems are in thermal equilibrium if they could transfer heat between each other, but don’t. Indeed, experiments have shown that if two systems, A and B, are in thermal equilibrium with each other, and B is in thermal equilibrium with a third system C, then A is also in thermal equilibrium with C. This conclusion may seem obvious, because all three have the same temperature, but zeroth law is basic to thermodynamics. Zeroth law justifies the use of thermodynamic temperature: the common “label” that the three systems in the definition above share is defined as the temperature of the systems.
Temperature
Thermometers actually take their own temperature, not the temperature of the object they are measuring. This raises the question of how we can be certain that a thermometer measures the temperature of the object with which it is in contact. The answer lies in the fact that any two systems placed in thermal contact (meaning heat transfer can occur between them) will reach the same temperature. That is, heat will flow from the hotter object to the cooler one until they reach exactly the same temperature. The objects are then in thermal equilibrium, and no further changes will occur. The systems interact and change because their temperatures differ, and the changes stop once their temperatures are the same. Thus, if enough time is allowed for this transfer of heat to run its course, the temperature a thermometer registers does represent the system with which it achieves thermal equilibrium.
Key Points
- Thermodynamic work is a generalization of the concept of mechanical work in mechanics.
- For closed systems, energy changes in a system other than as work transfer are as heat.
- Work in thermodynamics is a quite organized process (as in gas expansion), involving a macroscopic force exerted through a distance.
- The zeroth law of thermodynamics states that when systems, A and B, are in thermal equilibrium with each other, and B is in thermal equilibrium with a third system, C, then A is also in thermal equilibrium with C.
- Two systems are in thermal equilibrium if they could transfer heat between each other, but don’t.
- If enough time is allowed for heat transfer to occur between a thermometer and a system, the temperature the thermometer registers does represent the system with which it achieves thermal equilibrium.
Key Terms
- heat: energy transferred from one body to another by thermal interactions
- thermodynamics: a branch of natural science concerned with heat and its relation to energy and work
- internal energy: The sum of all energy present in the system, including kinetic and potential energy; equivalently, the energy needed to create a system, excluding the energy necessary to displace its surroundings.
- thermal equilibrium: Two systems are in thermal equilibrium if they could transfer heat between each other, but don’t.
- thermodynamic temperature: Temperature defined in terms of the laws of thermodynamics rather than the properties of a real material: expressed in kelvins
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Work (thermodynamics). Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Work_(thermodynamics). License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, College Physics. September 17, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42232/latest...n=col11406/1.7. License: CC BY: Attribution
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/de...nternal-energy. License: CC BY-SA: Attribution-ShareAlike
- heat. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/heat. License: CC BY-SA: Attribution-ShareAlike
- thermodynamics. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/thermodynamics. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, The First Law of Thermodynamics and Some Simple Processes. February 4, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42233/latest/. License: CC BY: Attribution
- Work. Located at: http://www.youtube.com/watch?v=q3svt4kUPdI. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- OpenStax College, Temperature. September 17, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42214/latest/. License: CC BY: Attribution
- Boundless. Provided by: Boundless Learning. Located at: www.boundless.com//physics/de...equilibrium--2. License: CC BY-SA: Attribution-ShareAlike
- thermodynamic temperature. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/thermo...ic_temperature. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, The First Law of Thermodynamics and Some Simple Processes. February 4, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m42233/latest/. License: CC BY: Attribution
- Work. Located at: http://www.youtube.com/watch?v=q3svt4kUPdI. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Celsius. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Celsius. License: CC BY: Attribution
- Thermodynamics and PV Diagrams. Located at: http://www.youtube.com/watch?v=aqQF_aX2naQ. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license


