1.7: Lipids in Non-Aqueous Environments
( \newcommand{\kernel}{\mathrm{null}\,}\)
It is widely known that in the presence of aqueous solutions, lipids will spontaneously form ordered structures such as micelles and bilayers. This process is driven by the structure of the lipid, consisting of an ionic polar group at the head of the lipid and a long hydrocarbon chain which is nonpolar. The head group of the lipid is hydrophilic, while the long nonpolar portion is hydrophobic or lipophilic. In the presence of water, a polar molecule, the structures organize themselves in order to minimize the exposure of the hydrophobic ends to the water molecules in the solution. This interaction is driven by the formation of hydrogen bonds and the arrangement of water around the polar head groups of the lipids (the formation hydration shells).

In the absence of water, the observed interactions between the lipids are free of the effects of the hydrogen bonding. These non-aqueous environments allow for the study of lipid behaviors in the absence of water.2 There are three main classifications of non-aqueous solvents used for the replacement of water in lipid bilayer systems: organic solvents, “dry” solvents, and purely ionic mediums. 2
Organic Solvents
Organic solvents are a non-aqueous class of liquid solvents which have a common structure containing at least one carbon and one hydrogen atom. They are generally lipophilic, allowing for their use in dissolving lipids. Organic solvents have been used to study the effect of water on lipid structures, specifically the structure and behavior of the lipids in the absence of water.
Glycerol is an organic solvent that has been used to substitute for water in the study of biological membranes; glycerol has been shown to substitute for water in biological systems. Multilamellar liposomes can be formed from phospholipids in pure glycerol. 3 Under pure glycerol conditions, the liposomes exhibit the same fluid space (fluid spacing between bilayers) that is measured in water. In solutions of glycerol and water, however, fluid spacing between the lipid bilayers is a function of the glycerol mole fraction in water due to glycerol changing the dielectric permittivity of fluid space. The dielectric constant of glycerol is around 42.5 compared to the dielectric constant of water of ~80. The effect of changing dielectric permittivity is to change the long-range Van der Waals forces between the lipid bilayers. 3
Interdigitated hydrocarbon chain packing
Alcohols, including ethanol, have been shown to have a biphasic effect on the gel to liquid-crystalline phase transition temperatures of saturated phosphatidylcholine bilayers. It is observed that at low concentrations of alcohol in solution, the lipids exhibit a decrease in Tm with increasing concentration. At higher alcohol concentrations there is a reversal in the slope of the changing Tm with respect to increasing concentration of alcohol; at high concentrations there in an increase of Tm with increasing alcohol concentration. 4,5
The concentration at which the reversal takes place is also dependent on the length of the hydrocarbon tails of the lipids; it is more energetically favorable for long chain lipids to interdigitate than short chained lipids due to Van der Waals interactions. The decrease in Tm in the low concentration ranges is explained by freezing point depression as a result of the preferential interaction of the alcohol with the liquid-crystalline phase of the lipid. The reversal in the trend at higher concentrations of alcohol is due to an induced gel phase in which the phospholipids from opposing monolayers interdigitate/interlock.

"Dry" Solvents
It is possible to maintain the integrity of lipid membranes in the absence of water using certain molecules. These molecules, usually a sugar such as trehalose, serve to act as substitutes for water in the structure of the lipid bilayers. 6
“Wet” Interactions
The roles dry solvents play in interacting with phospholipids can best understood in the context of the interactions water has with the lipids. There are several things to consider regarding the interactions of water with these lipids, namely the affects of hydration such as shell formation, swelling, and headgroup separation between bilayers. For example, when water hydrates phospholipids, the lipids swell as water becomes bound to the headgroup of the lipids. Phosphatidylcholines can bind 10-12 mol of water per mol of lipid. 7 The water which binds to the lipids forms the hydration shell of the lipid. Increase in hydration of phospholipid bilayers can result in increased area per lipid molecule, increased disorder among hydro carbon chains, and increased lamellar repeat distances. The lamellar repeat distance is the separation of head groups between stacked lipid bilayers.
Decreased hydration levels (generally below 20%) result in increased van der Waal’s interactions, which increase the likelihood of the lipid undergoing a liquid crystalline to gel phase transition. 7
Dehydration
Dehydration of lipid bilayers can result in fusion. Dehydration causes a reduction in the force of hydration which opposes the fusion of lipid bilayers. Fusion of lipid bilayers during dehydration has been shown using freeze-fracturing techniques. In the cases where fusion has been observed, the fusion is between adjacent bilayers. 7 The implications of this fusion are evident in cellular membranes that undergo dehydration. Fusion of cellular membranes can result in leakages during the transitions from liquid-crystalline to gel phase (drying) and gel to liquid-crystalline (rehydration).

Sugar Protectants
Membranes may be stabilized in the absence of water in presence of dry sugars such as trehalose. The sugars are theorized to stabilize lipid bilayers by inhibiting the fusion of the lipids in the absence of water. In the case of trehalose, the addition of small amounts of the dry solvent (~1g trehalose/g phospholipid) preserves phospholipids in the “dry” state while maintaining the phospholipid's liquid crystalline phase. High resolution proton NMR can be used to demonstrate that phospholipids dried without a dry/stabilizing sugar present result in gelling of the hydrocarbon chains, while in the presence of trehalose the hydrocarbon chains remain in a fluid phase. 7
There is likely a direct interaction between the phosphate head groups of the lipid and the hydroxyl groups of the trehalose due to hydrogen bonding. Other sugars such as sucrose, which are not as readily able to form hydrogen bonds, can also be used with varying degrees of success (Figure 1.7.4).

Purely Ionic Solvents
Purely ionic salts, also known as fused salts are unique solvents in that they are able to provide purely ionic medium in the absence of water. Thus, these solvents can be used to study the role of ionic bonding between the phosphate head groups of phospholipids and the corresponding interactions resulting from hydrogen bonding in water. 8 Similar to the organic solvents and dry solvents, the fused salt is used to replace water in studies concerning phospholipid bilayers.
Ethylammonium nitrate
Ethylammonium nitrate (EAN) is one of the earliest identified room temperature ionic liquids with a melting point of 12 degrees C. The ionic fluid has been used to study the interactions of distearoylphospatidylcholine (DSPC) and L-dipalmitoylphospatidylcholine (DPPC). 9 The presence of EAN had little effect in the phase transition of DSPC, however in subsequent studies with DPPC in the presence of EAN it has been observed that a subgel to liquid crystalline transition is observed. The subgel transition is unique to the purely ionic solvents. In the interaction of the ionic fluid with the lipids, the acyl chains are disordered, forcing a transformation from the subgel bilayer to a Hexagonal array of cylinders (HII) without passing through L-beta, L-beta', or L-alpha intermediate forms. 9
Additionally, the acyl chain packing structure (also known as subcells) for DPPC in EAN were found to be larger than the DPPC subcells in water. 8 The implications of these observations in EAN may be that the gel-phase observed in water is characteristic of the water's interaction with the lipid, but not the ionic fluid's; the gel phase may only be a metastable phase. 9 In the case of the presence of the ionic fluid, the larger DPPC subcells may allow for the thermodynamically stable Hexagonal array to be the high-temperature liquid crystalline phase.
The evidence of these phase transitions is shown below in the following differential scanning calorimetry thermogram. In (a) the DPPC in EAN undergoes a transition from L-c to an H-alpha or some non lamellar phase.(b) Initial cooling results in a transition from the non lamellar phase to a gel phase. It is unlikely that during cooling the process would result in the formation of an L-c phase. (c) The reheating results in two phase transitions possibly from the L-c to the L-alpha phase and then from the L-alpha to the Hexagonal array phase.

Liquid Crystal Formation
Liquid crystal formation of DSPC in the presence of totally ionic fluid EAN can be viewed by polarizing microscopy. In a 1:1 mixture of DSPC to EAN was micrographed in the range of 57 to 100 degrees C. In this range the micrographs show a texture of oily streaks. Below a measured temperature of 56.7 degrees, the oily streaks take on the appearance of rigid, angular bundles. 9

Figure 1.7.6: Micrograph of DSPC - EAN (1:1 mixture), T=57.6 C. Note "oily streak" appearance 9
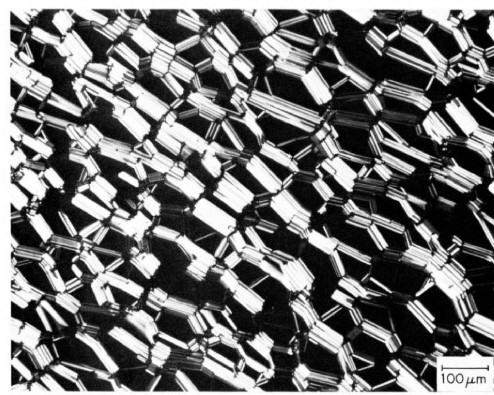
Figure 1.7.7: Micrograph of DSPC - EAN (1:1 mixture), T=56.1 C. Note rigid bundle appearance 9
The evidence of these two phase transitions are analogous to well-known order-disorder or gel-liquid crystalline transitions that are observed in lecithin-water mixtures. 9
Summary
The three main classifications of non-aqueous environments with applications to the study of lipids are organic solvents, dry solvents, and purely ionic salts. Each of these environments give insight into the behavior of lipids in the absence of water, providing invaluable information regarding the importance of water on the structure of lipids. Organic solvents are generally used to dissolve lipids. In the case of some solvents, like alcohols, the solvent can replace water in the fluid space between lipids, influencing the interactions and spacing between the lipid bilayers. Dry solvents can come in the form of dry sugars and other molecules. These sugars serve to protect lipid membranes by preventing fusion via hydrogen bond interactions with the hydrophilic headgroups of the phospholipid. Fused salts are used to determine the influence of purely ionic interactions in the the absence of hydrogen bonding. Each of these environments are used to create simple models systems for studying the phase changes, the bilayer structure, thermal behaviors, and interaction forces between bilayers. Practical applications of the study of these environments are numerous, including the preservation of the lipid membranes of cells during drying/rehydrating. 7
References
1. Lipids | Microbiology. https://courses.lumenlearning.com/mi...hapter/lipids/. Accessed May 15, 2019.
2. Lis, L J;Tamura-Lis W. Structures and Mechanisms of Lipid Phase Transitions in Nonaqueous Media : Dipalmitoylphosphatidylcholine in Fused Salt. 1987;(6):4625-4627. doi:10.1021/j100301a039
3. McDaniel R V., McIntosh TJ, Simon SA. Nonelectrolyte substitution for water in phosphatidylcholine bilayers. BBA - Biomembr. 1983;731(1):97-108. doi:10.1016/0005-2736(83)90402-9
4. Simon SA, McIntosh TJ. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. BBA - Biomembr. 1984;773(1):169-172. doi:10.1016/0005-2736(84)90562-5
5. McIntosh TJ, McDaniel R V., Simon SA. Induction of an interdigitated gel phase in fully hydrated phosphatidylcholine bilayers. BBA - Biomembr. 1983;731(1):109-114. doi:10.1016/0005-2736(83)90403-0
6. Crowe, Lois M.;Womersley, Christopher;Crowe, John H.; Reid, David; Appell, Lori; Rudolph A. Prevention of fusion and leakage in freeze-dried liposomes by carbohydrates. Biochemistry. 1986;861:131-140.
7. Crowe JH, Crowe LM, Carpenter JF, Wistrom CA. REVIEW ARTICLE Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242:1-10. https://www.ncbi.nlm.nih.gov/pmc/art...00261-0011.pdf.
8. Leary TJC, Levin IW. Effects of solvent on biomembrane structure: Raman spectroscopic investigation of dipalmitoylphosphatidylcholine dispersed in N-ethylammonium nitrate. J Phys Chem. 1984;88(18):4074-4078. doi:10.1021/j150662a044
9. Evans DF, Kaler EW, Benton WJ. Liquid crystals in a fused salt: .beta.,.gamma.-distearoylphosphatidylcholine in N-ethylammonium nitrate. J Phys Chem. 1983;87(4):533-535. doi:10.1021/j100227a003

