1.1: Charged Lipids
- Page ID
- 1358
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Lipids comprise a diverse group of compounds that include fats, waxes, sterols, phospholipids and many others. The functions associated with lipids are just as diverse, and include membrane structure, signaling, and energy storage. While most lipids are composed of non-polar hydrocarbon structures, other lipids can contain positively and/or negatively charged elements, the nature of which imparts particular physical properties that give charged lipids structural and functional versatility. This page will describe the structure and function of some of the charged lipids commonly encountered in biology.
Types of charged lipids
Fatty acids
The simplest of the charged lipids, fatty acids are a large group of amphipathic molecules consisting of short, medium or long-chain hydrocarbon “tails” (C4 to C36) and a polar carboxylic acid “head”. The aliphatic chains can be fully saturated or unsaturated to some extent, and provide the hydrophobic character of the fatty acid. Regardless of the length of the aliphatic tail group the hydrophilic carboxylic acid group will have a pKa around 4.0, which means that under physiological conditions, the majority of fatty acids will have a negative charge of -1. The tails can also contain carbon ring structures, hydroxyl groups, and additional methyl group branches (Figure \(\PageIndex{1}\)).
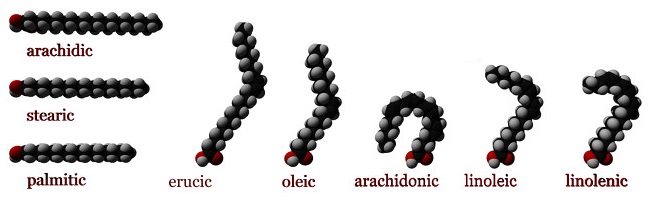
The physical properties of fatty acids are largely dependent on the length and degree of unsaturation of the hydrocarbon tail group. The major factor influencing properties such as melting point and water solubility is the ordering of water molecules around the hydrophobic tails. Fatty acids will cluster together as result of the hydrophobic effect. The clustering of the aliphatic tail groups forms a crystalline lattice, minimizing the exposed hydrophobic surface area and the formation of a hydration shells around individual fatty acid tails. With increasing degrees of unsaturation in the tails (increased number of double bonds), the effect is reversed. Unsaturation introduces kinks into the tails so that they no longer pack as uniformly and tightly, which allows water molecules to become ordered around the individual tails. As such, the crystalline lattice is weakened, increasing the solubility of the fatty acid and decreasing its melting point.

Fatty acids have a variety of important roles. The hydrocarbon tails are a major source of energy when each successive two-carbon unit is converted into acetyl-CoA during beta-oxidation, the process of which generates NADH and FADH2 which are subsequently used in the formation of ATP in oxidative phosphorylation. Fatty acids and their derivatives also function as soaps and detergents. At high enough concentrations, fatty acids will aggregate to form spherical structures called micelles, which are energetically favorable structures that increase the entropy of water by minimizing water clathrate formation around the hydrophobic regions. They accomplish this by clustering the hydrophobic tails into the center of the sphere, while the hydrophilic heads form a shell that is water soluble. The spherical structure is favored by fatty acids because the cross-sectional area of the head group is larger than that of the tail, forming a conical shape. When soaps (the salts of fatty acids) are mixed with water, the micelles that are formed sequester oils and hydrophobic “dirt” into the micelle center, while the hydrophilic outer shell helps to wash the dirt-carrying micelles away. Lastly, fatty acids form the foundation of di- and triacylglycerols, a family of molecules with two or three fatty acid chains bound by glycerol. When the third hydroxyl group of the glycerol is bound to a charged molecule such as phosphate instead of a hydrocarbon chain, the diacylglycerol is known as a phospholipid.
Phospholipids
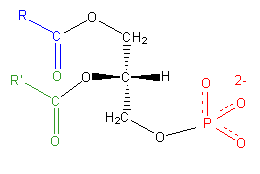
As with any molecule, the formation of a charge requires the inclusion of an ionizable element, most often covalently bound to the parent compound. In the case of diacylglycerols, non-ionizable hydrocarbons require the covalent addition of an ionic group to the glycerol to impart a charge. By far the most common of these is the negatively charged phosphate group (\(PO_4^{3-}\)) which, when covalently bound to the glycerol moiety of a two-chain fatty acid, forms the main group of charged fatty acids known as phospholipids. Phospholipids are amphipathic molecules composed of three sections: a diglycerol, a hydrophilic “head” consisting of the charged phosphate moiety, and a small organic molecule covalently bound to the phosphate (Figure \(\PageIndex{3}\)).

Phospholipids comprise the major components of lipid bilayers in cellular membranes. Similar to fatty acids, phospholipids will favor the clustering of the hydrophobic tails to exclude water as much as possible. Unlike fatty acids, however, the presence of two tails favors the formation of a more planar membrane because the cross-sectional area of the head and tail groups is similar, forming a cylindrical shape which is most efficiently packed as a bilayer. In the bilayer, the hydrophobic tails interact with one another, whereas the hydrophilic head groups interact with the aqueous environment on either side of the bilayer. While the bilayer is most practical as a two dimensional planar structure, it can fold in on itself to form a closed, vesicle called a liposome. Unlike the micelle, the liposome has a bilayer wall enclosing a hollow, aqueous cavity, and is used organisms as a transport mechanism for any number of substances.
The structural complexity of the membrane bilayer goes beyond a single type of phospholipid molecule. There exist a variety of phospholipids with varying head groups and hydrocarbon tails. Additionally, membrane lipids are not symmetrically distributed throughout the membrane bilayer, whereby certain phospholipids are more likely to be found in the inner monolayer with the others more likely to be found in the outer leaflet. On the other hand, the physical properties of the different head groups dictate the interactions of each phospholipid with the aqueous environment, and resulting in the movement of the lipids across the bilayer in both the vertical and horizontal directions, continuously changing its shape and phospholipid organization. To give a better idea of the different phospholipid head groups, the following sections will describe the structural features some of the more common ones.
Types of Phospholipids
Phosphatidic acid
As the sole member of the head group, the phosphate imparts a -2 charge to the lipid molecule and is referred to as phosphatidic acid (PA). The phosphate moiety exhibits several different levels of protonation, depending on the pH. Below a pH of 4.0, it carries a charge of zero, whereas above pH 12, it is fully deprotonated and with a -2 charge. Thus, it goes to reason that at physiological pH, the phosphate group is only partially protonated, carrying a -1 charge, and imparting a net charge of -1 to the PA molecule.
PA's greatest role is in the biosynthesis of triacylglycerols and other phospholipids, as its structure serves as the backbone these compounds. Although PA is the simplest of all the phospholipids, it occurs only in small amounts in biological membranes. Because of the unique ionization properties of its head-group, which can deprotonate to a -2 charge in the process of forming an intermolecular hydrogen bond, it serves a crucial role in the binding of basic protein and small molecule compounds (2). This property not only makes PA an important phospholipid for protein-membrane docking, but also has implications in membrane dynamics and cellular signaling.
Phosphatidylcholine
Phosphatidylcholine (PC) adds an additional positively-charged group, choline, to the phosphate head group of phosphatidic acid. Choline is an N,N,N-trimethylethanolammonium cation and an essential nutrient required for membrane structural integrity, signaling, and neurotransmission. Once choline binds to phosphatidic acid, the phosphate group loses one of its negative charges, making PC an overall neutral molecule: a positive charge on the choline and a negative charge on the phosphate. The nature of the hydrocarbon tails determines the type of phosphatidylcholine molecule, where one tail is usually an unsaturated hydrocarbon such as oleic acid and the other a fully saturated one.
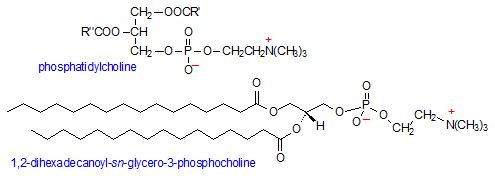
PC is one of the more abundant phospholipids in cellular membranes, where it can comprise up to 50% of the total membrane phospholipid composition and is found primarily in the outer leaflet of membranes. PC's other major role is as an integral component of plasma lipoproteins, where it accounts for 60-80 mol% of total phospholipids. (3)
Sphingomyelin
Similar to phosphatidylcholine, zwitterionic sphingomyelin contains the phosphocholine head group. Unlike PC and other phospholipids containing glycerol, however, the sphingomyelin backbone is an unsaturated 18-carbon amino-alcohol group called sphingosine. An additional hydrocarbon chain, typically of a different length, is attached to the other sphingosine hydroxyl group.
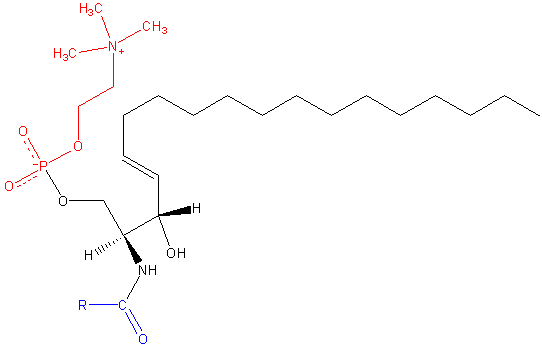
Next to PC, sphingomyelin (SM) is the most abundant charged lipid, and the most abundant sphingolipid, found in the outer leaflet of cellular membranes. SMs are important molecules in membrane fluidity, as they are heavily involved in the formation of lipid rafts and ordered domains on the membrane surface, binding to membrane-spanning proteins, and are involved in cellular signaling and membrane homeostasis. (4) SMs come in a variety of long-chain bases depending tissue distribution, with the most common one being sphingosine (pictured above). Others include palmitic acid, a common N-linked acyl chain of SM in mammalian peripheral cells, and stearic acid, which is more common to neuronal tissues.
Phosphatidylethanolamine
Phosphatidylethanolamine (PE) is the second most abundant phospholipid and is comprised of the typical phosphatidic acid with an additional head group component, namely ethanolamine. Ethanolamine is both a primary amine and alcohol, and attaches to the PA head group via the hydroxyl. Because the amino group has a pKa of about 9.5, it is protonated under physiological pH, making PE a zwitterionic and neutral molecule.
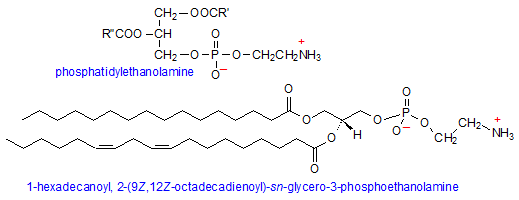
While PE is just as (if not more) abundant in lipid membranes as PC and SMs, it prefers to occupy the inner leaflet of the membrane, facing the cytoplasm. Because of its smaller head group, the molecule takes on a conical shape which can exert lateral pressure on the membrane, thus modulating its curvature and stabilizing membrane proteins. (5) Additionally, the positive charge on the head group is able to bind to a variety of signaling proteins and balance local negative charge on the membrane.
Phosphatidylserine
Phosphatidylserine (PS) is composed of phosphatidic acid (PA), with the negatively charged phosphate group attached to the amino acid serine at the hydroxyl end. Because of the carboxyl and amino groups on serine, in addition to the negatively charged phosphate group, it is possible for PS to have net charge of -1. This is because the carboxyl and amino groups of serine have pKas of 2.21 and 9.15, respectively, so that the carboxyl group will be have a -1 charge at physiological pH; the reverse will be true for the amino group.
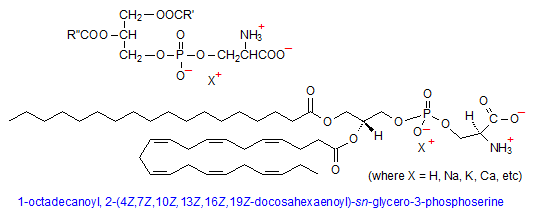
Phosphatidylserine comprises only about 10% of total cell membrane phospholipids and is found almost exclusively in the inner monolayer, most prominently in myelin of brain tissue. One of the most prominent functions of PS is in blood coagulation, whereby its transport to the outer membrane layer in activated platelets is thought to either enhance the rate of prothrombin formation, or to allosterically alter the proteolytic and cofactor capabilities of prothrombin factors Xa and Va in thrombin activation. (6) In a completely different role, exposure of PS to the extracellular side of a membrane is often a cellular marker for apoptosis, or programmed cell death. (7)
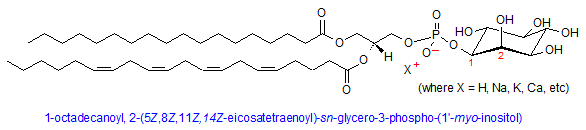
Phosphatidylinositol
Phosphatidylinositol (PI) is a unique phospholipid in that the head group is a cyclohexane carbohydrate called inositol, which is attached to the phosphate moiety at the 1’ carbon. Unique to inositol is the ability to become phosphorylated at the 3’, 4’, and 5’ carbons by a host of PI-kinases, which can add up to three phosphate groups at those positions, per molecule. This phenomenon can impart up to a -4 charge on the PI molecule. Although comprising only about 5% of total lipid content in membranes, phosphoinositols (PIs) are concentrated on the inside leaflet of the lipid bilayer where they play crucial roles in cellular signaling, protein binding, and membrane dynamics. (8)
References
- David L. Nelson & Michael M. Cox, "Principles of Biochemistry, fourth edition." W. H. Freeman and Company, New York. 2005
- Edgar Eduard Kooijman & Koert N. J. Burger (2009). "Biophysics and function of phosphatidic acid: A molecular perspective". Biochimica et Biophysica Acta 1791 (9): 881-888.
- Cole, L.K., Vance, J.E., Vace, D.E. (2012). "Phosphatidylcholine biosynthesis and lipoprotein metabolism". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1821 (5): 754-761.
- Petter J. Slotte (2013). "Molecular properties of various structurally defined sphingomyelins – correlation of structure with function". Progress in Lipid Research 52 (2): 206-219.
- Suetsugu, S., Kurisu, S., Takenawa, T. (2014). "Dynamic Shaping of Cellular Membranes by Phospholipids and Membrane-Deforming Proteins". 94 (4):
- . "Exposure of platelet membrane phosphatidylserine regulates blood coagulation". Progress in Lipid Research 42 (5): 423-428.
- Peter A. Leventis & Sergio Grinstein (2010). "The Distribution and Function of Phosphatidylserine in Cellular Membranes". Annual Reviews of Biophysics 39: 407-27
- Gilbert Di Paolo & Pietro De Camilli (2006). "Phosphoinositides in cell regulation and membrane dynamics". Nature 443: 651-657.

