11.6: Preventing Hypothermia
- Page ID
- 17807
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hypothermia
| Stage | Core Body Temperature °C | Symptoms |
| Mild Hypothermia | 35°-33° | shivering, poor judgment, amnesia and apathy, increased heart and respiratory rate, cold and/or pale skin |
| Moderate Hypothermia | 32.9°-27° | progressively decreasing levels of consciousness, stupor, shivering stops, decreased heart and respiratory rate, decreased reflex and voluntary motion, paradoxical undressing. |
| Severe Hypothermia | < 26.9° | low blood pressure and bradycardia, no reflex, loss of consciousness, coma, death |
Thermal Power
The rate at which chemical potential energy is converted to thermal energy by the body (and other systems) is the thermal power. When the thermal power is less than than the heat loss rate then the body will lose thermal energy over time and body temperature will drop. The only options for preventing hypothermia are slowing down the heat loss rate and/or increasing the thermal power. You can fight off hypothermia by doing additional work, such as jumping around, because the body is inefficient so most of the chemical potential energy used to do the work actually becomes thermal energy that can replace what was lost as heat. Shivering is your body’s way of forcing you to take this approach and signifies a mild stage of hypothermia. However, this strategy will only be successful until you have used up your readily accessible supply of chemical potential energy. Basically, as you get tired this method will fail. The overall chemical to thermal energy conversion rate can be supplemented by technology such chemical hand/foot warmers and battery powered heated clothing, but in most situations will your body does the bulk of the conversion. Eventually these supplemental energy sources will also run out and body temperature will continue to drop. Moderate hypothermia is indicated by the end of shivering and increased mental confusion, possibly including hallucinations. Severe hypothermia leads to loss of consciousness and if not treated, eventually death.[2]
Everyday Example: Human Thermal Power
The typical daily intake of chemical potential energy required by the human body is 2000 Calories. A hard 8 hours of manual labor only accounts for 1/3 of a day and during the other 2/3 almost no useful work is done by the body so nearly all chemical energy being used is converted to thermal energy. Even when useful work is being done, the body is only about 25% efficient so most of the chemical energy used is still converted to thermal energy. Therefore we can reasonably approximate the thermal power (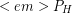 P_H" title="Rendered by QuickLaTeX.com" height="13" width="72" style="vertical-align: -2px;">) of the human body to be roughly 2000 Calories/day by assuming all chemical energy used eventually becomes thermal energy. Remembering that food Calories with a capitol C are actually kcals and that 4.186 Joules are in one calorie, we can use unit conversion to find the thermal power in SI units of Watts.
P_H" title="Rendered by QuickLaTeX.com" height="13" width="72" style="vertical-align: -2px;">) of the human body to be roughly 2000 Calories/day by assuming all chemical energy used eventually becomes thermal energy. Remembering that food Calories with a capitol C are actually kcals and that 4.186 Joules are in one calorie, we can use unit conversion to find the thermal power in SI units of Watts.

Preventing Hypothermia
Your body loses heat to the environment due to a natural tenancy of systems to move toward thermal equilibrium. In fact the Second Law of Thermodynamics tells us that objects left to themselves will always spontaneously trend toward thermal equilibrium with their environment. For two objects to reach thermal equilibrium, heat must transfer away from the hot object and into the cold one so that their temperatures move closer together. Therefore, a consequence of the Second Law of Thermodynamics is that heat will always spontaneously transfer from warmer temperature to colder temperature. Homeostasis is a constant battle against the consequences of the Second Law of Thermodynamics. We aren’t able to violate the second law of thermodynamics and stop or reverse the spontaneous thermal energy transfer away from the body in cold environments, we can only try to slow it down.
Reinforcement Exercises
If you add equal parts of 10 °C water and 80 °C water, will you get 90 °C water?
Explain your answer in terms of the Second Law of Thermodynamics.
Also explain your answer in terms of the definition of temperature as a measure of the average kinetic energy per molecule.
Heat Transfer
Materials designed to slow the heat transfer rate, or thermal insulation, can be used to help prevent hypothermia. There are three ways that heat is transferred out of the body, but all three methods follow the Second Law of Thermodynamics and transfer heat from warmer temperature to colder. The heat transfer mechanisms are:
- Conduction
- Convection
- Radiation
The following chapters will discuss these mechanisms and the types of insulation used to prevent each.
Everyday Examples: Insulation
My father was a bush pilot in Alaska. When I was about 13 years old we were landing on a lake in our hometown and found two teenagers clinging to their overturned canoe. The first boy had a stocky build and second was tall and thin. The first boy climbed onto the float and into the plane with some assistance, the thin boy was unable to move and was dragged out of the water just before losing consciousness as we rode back to shore. We later learned that the thin boy had reached the third stage of hypothermia and was likely only minutes from death. The thin boy had less body mass, thinner layers of tissue to provide insulation, and less chemical potential energy stored up for conversion to thermal energy. Both boys were wearing cotton clothing, which did not provide much insulating value in the water. In the following chapters we will learn how each of these factors contributed to the dramatically different in responses of the two boys to their unplanned cold water immersion.
- Adapted from "Web-based hypothermia information: a critical assessment of Internet resources and a comparison to peer-reviewed literature" by M Spencer, Jeremy & Sheridan, Scott, Perspectives in public health, 135(2) · February 2014↵
- "Hypothermia and Cold Related Injuries" by J. Justad, MD, DDP, Health and Safety Guidelines, Montana Department of Health and Human Services↵


