8.8: The First Law of Thermodynamics
( \newcommand{\kernel}{\mathrm{null}\,}\)
Learning Objectives
- Define the first law of thermodynamics.
- Describe how conservation of energy relates to the first law of thermodynamics.
- Identify instances of the first law of thermodynamics working in everyday situations.

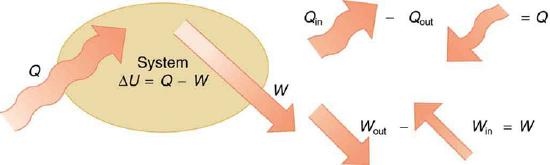
If we are interested in how heat transfer is converted into doing work, then the conservation of energy principle is important. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. In equation form, the first law of thermodynamics is
ΔU=Q−W.
Here ΔU is the change in internal energy U of the system. Q is the net heat transferred into the system—that is, Q is the sum of all heat transfer into and out of the system. W is the net work done by the system—that is, W is the sum of all work done on or by the system. We use the following sign conventions: if Q is positive, then there is a net heat transfer into the system; if W is positive, then there is net work done by the system. So positive Q adds energy to the system and positive W takes energy from the system. Thus ΔU=Q−W. Note also that if more heat transfer into the system occurs than work done, the difference is stored as internal energy. Heat engines are a good example of this—heat transfer into them takes place so that they can do work. (See Figure 8.8.2.) We will now examine Q, W, and ΔU further.
MAKING CONNECTIONS: LAW OF THERMODYNAMICS AND LAW OF CONSERVATION OF ENERGY
The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The first law gives the relationship between heat transfer, work done, and the change in internal energy of a system.
Heat Q and Work W
Heat transfer (Q) and doing work (W) are the two everyday means of bringing energy into or taking energy out of a system. The processes are quite different. Heat transfer, a less organized process, is driven by temperature differences. Work, a quite organized process, involves a macroscopic force exerted through a distance. Nevertheless, heat and work can produce identical results.For example, both can cause a temperature increase. Heat transfer into a system, such as when the Sun warms the air in a bicycle tire, can increase its temperature, and so can work done on the system, as when the bicyclist pumps air into the tire. Once the temperature increase has occurred, it is impossible to tell whether it was caused by heat transfer or by doing work. This uncertainty is an important point. Heat transfer and work are both energy in transit—neither is stored as such in a system. However, both can change the internal energy U of a system. Internal energy is a form of energy completely different from either heat or work.
Internal Energy U
We can think about the internal energy of a system in two different but consistent ways. The first is the atomic and molecular view, which examines the system on the atomic and molecular scale. The internal energy U of a system is the sum of the kinetic and potential energies of its atoms and molecules. Recall that kinetic plus potential energy is called mechanical energy. Thus internal energy is the sum of atomic and molecular mechanical energy. Because it is impossible to keep track of all individual atoms and molecules, we must deal with averages and distributions. A second way to view the internal energy of a system is in terms of its macroscopic characteristics, which are very similar to atomic and molecular average values.
Macroscopically, we define the change in internal energy ΔU to be that given by the first law of thermodynamics:
ΔU=Q−W.
Many detailed experiments have verified that ΔU=Q−W, where ΔU is the change in total kinetic and potential energy of all atoms and molecules in a system. It has also been determined experimentally that the internal energy U of a system depends only on the state of the system and not how it reached that state. More specifically, U is found to be a function of a few macroscopic quantities (pressure, volume, and temperature, for example), independent of past history such as whether there has been heat transfer or work done. This independence means that if we know the state of a system, we can calculate changes in its internal energy U from a few macroscopic variables.
MAKING CONNECTIONS: MACROSCOPIC AND MICROSCOPIC
In thermodynamics, we often use the macroscopic picture when making calculations of how a system behaves, while the atomic and molecular picture gives underlying explanations in terms of averages and distributions. We shall see this again in later sections of this chapter. For example, in the topic of entropy, calculations will be made using the atomic and molecular view.
Section Summary
- The first law of thermodynamics is given as ΔU=Q−W, where ΔU is the change in internal energy of a system, Q is the net heat transfer (the sum of all heat transfer into and out of the system), and W is the net work done (the sum of all work done on or by the system).
- Both Q and W are energy in transit; only ΔU represents an independent quantity capable of being stored.
- The internal energy U of a system depends only on the state of the system and not how it reached that state.
Glossary
- first law of thermodynamics
- states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system
- internal energy
- the sum of the kinetic and potential energies of a system’s atoms and molecules
- human metabolism
- conversion of food into heat transfer, work, and stored fat


