12.3: The Photoelectric Effect
( \newcommand{\kernel}{\mathrm{null}\,}\)
Learning Objectives
- Explain features of photoelectric effect.
- Explain why photon hypothesis is necessary to explain the observed features in photoelectric effect experiments.
When light strikes a conductor surface ("photocathode"), it can eject electrons from them. This is called the photoelectric effect, meaning that light (photo) produces electricity. One common use of the photoelectric effect is in light meters, such as those that adjust the automatic iris on various types of cameras. In a similar way, another use is in solar cells, as you probably have in your calculator or have seen on a roof top or a roadside sign. These make use of the photoelectric effect to convert light into electricity for running different devices.
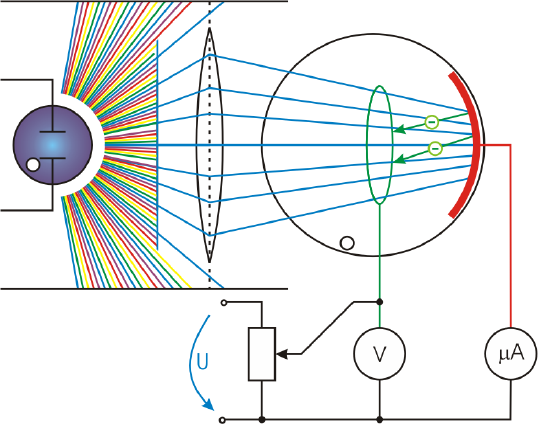

This effect has been known for more than a century and can be studied using a device schematically shown in Figure 12.3.1. This figure shows an evacuated tube with a metal plate and a collector wire that are connected by a variable voltage source, with the collector more negative than the plate. When light strikes the plate in the evacuated tube, it may eject electrons ("photoelectrons"). If the photoelectrons have kinetic energy greater than the potential energy needed to reach the negative-voltage collector wire, some electrons will be collected on the wire. Using this, the maximum electron kinetic energy can be measured by adjusting the retarding voltage between the wire and the plate. For example, if −3.00 V barely stops the electrons, then the electrons needed to overcome potential energy difference of ΔPE=(−e)(−3.00 V)=3.00 eV. This means the kinetic energy of most energetic photoelectrons was 3.00 eV. The number of photoelectrons can also be determined by measuring the current between the wire and plate ("photocurrent"). Often, there is a direct linear relationship between the intensity of light and the number of photoelectrons and the amount of photocurrent. With a current amplifier, this can be used as a light meter.
As people studied the photoelectric effect, following properties became evident. For simplicity, these are properties shown when monochromatic (single wavelength, or frequency) EM radiation is incident on a photocathode.
- If we vary the frequency of the EM radiation falling on a material, we find the following: For a given material, there is a threshold frequencyf0 for the EM radiation below which no electrons are ejected, no matter how intense the EM radiation.
- Once EM radiation falls on a material, electrons are ejected without a measurable delay.
- The number of electrons ejected per unit time is proportional to the intensity of the EM radiation, provided that the frequency of the EM radiation is high enough to eject photoelectrons.
- If we measure the kinetic energy of ejected electrons as a function of experimental parameters, we find the following: the maximum kinetic energy of ejected electrons is dependent on the frequency of the EM radiation but not on the intensity of the EM radiation.
Some of these features are easy to understand from the classical understanding of EM radiation as electromagnetic wave. Larger intensity of EM radiation means larger amplitude of electromagnetic wave, which would cause more electrons to be ejected. But some of the features, especially the existence of threshold frequency, could not be explained using the classical theory of electromagnetic wave. Why is it not possible to make up for reduced frequency by increasing the intensity of EM radiation? It took the genius of Albert Einstein to realize that he can use the quantization of energy idea introduce by Max Planck to explain this. Einstein realized that these characteristics of the photoelectric effect could be fully explained if EM radiation is itself quantized: the apparently continuous stream of energy in an EM wave is composed of energy quanta called photons. In his explanation of the photoelectric effect, Einstein defined a quantized unit or quantum of EM energy, which we now call a photon, with an energy proportional to the frequency of EM radiation. In equation form, the photon energy is
E=hf,
where E is the energy of a photon of frequency f and h is Planck’s constant.
This is a revolutionary extension of Planck's original quantization of energy. It is an extension, because Planck never claimed EM radiation itself was quantized, only the energy levels in thermal oscillators. It is revolutionary because physicists had thought they understood EM radiation completely as an electromagnetic wave, fully described by Maxwell's equations. Physicists had debated intensely a century earlier on whether light was a particle (Sir Isaac Newton advanced a corpuscular theory of light) or a wave; experimental evidences convinced nearly everyone that light was a wave; and this achievement in classical physics was capped off by James Clerk Maxwell's unified theory of electromagnetism, predicting existence of an electromagnetic wave, which turned out to be light. Should we now go back to a particle theory of light?
This is not an easy question to answer. All the experimental evidences that suggested that light is an electromagnetic wave are still true. On the other hand, the photoelectric effect cannot be explained by clinging to the classical wave theory of light. This is the beginning of quantum mechanics, and a concept we will call "wave-particle duality." We should not be too quick to answer this question. Quantum mechanics is not an easy topic to understand, not just mathematically but also conceptually. All the brilliant physicists we will talk about were wrong at one point or another about some aspect of quantum mechanics. Even Einstein himself was wrong when he said "God does not play dice," in frustration over probabilistic, non-deterministic aspects of quantum mechanics. Our goal is for you to become aware of quantum mechanical concepts and effects; it is perfectly fine not to understand everything in quantum mechanics right away. Richard Feynman (another hero in modern physics) famously said once, "If you think you understand quantum mechanics, you don't understand quantum mechanics."
In the meantime, this is how Einstein’s idea that EM radiation is quantized explains the features of photoelectric effect (we will re-visit wave-particle duality, or particle-wave duality, later).
- Threshold frequency is explained by individual photons interacting with individual electrons. Thus if the photon energy is too small to break a single electron away, no electrons will be ejected. (Note that if EM radiation interacts as a wave, sufficient energy could be provided by increasing the intensity of EM radiation.)
- There are no measurable delays, because as soon as an individual photon of a sufficiently high frequency (and thus sufficiently high energy) is absorbed by a single electron, the electron is ejected. Even at a very low intensity, a single photon of enough frequency would have enough energy to eject a single electron. It is difficult to see how this could be the case, if the EM radiation interacts as a wave. For a classical wave, at some very low intensity, some measurable amount of time would be required to transfer enough energy.
- The number of photoelectrons increasing with intensity is easy to see both under the particle and wave model of light, as mentioned before.
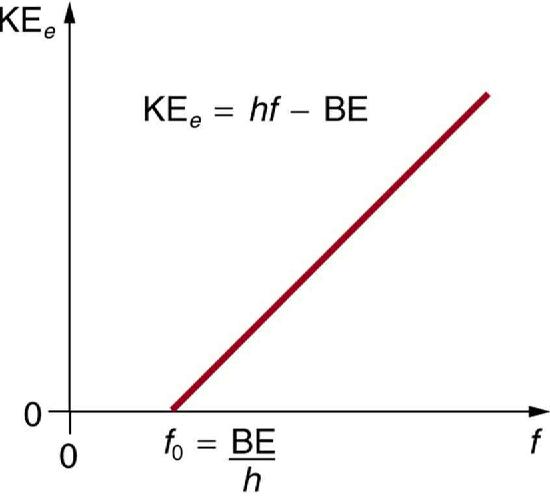
- The kinetic energy (KEe) of an ejected electron equals the photon energy minus the binding energy (BE) of the electron in the specific material, which is called the work function. Taking all of the photon energy (hf) to the electron, some of it is used to break the electron away from the material. The remainder goes into the ejected electron’s kinetic energy. In equation form, this is expressed as:
KEe=hf−BE.
This equation, which Einstein proposed in 1905, explains the properties of the photoelectric effect quantitatively. The binding energy can be determined in an experiment that determines the threshold frequency f0 for the material. The binding energy is BE=hf0. Figure 12.3.4 shows a graph of maximum KEe versus the frequency of incident EM radiation falling on a particular material, which would be used to estimate the threshold frequency.
Example 12.3.1: Calculating Photon Energy and the Photoelectric Effect: A Violet Light
(a) What is the energy in joules and electron volts of a photon of 420-nm violet light? (b) What is the maximum kinetic energy of electrons ejected from calcium by 420-nm violet light, given that the binding energy (or work function) of electrons for calcium metal is 2.71 eV?
Strategy
To solve part (a), note that the energy of a photon is given by E=hf. For part (b), once the energy of the photon is calculated, it is a straightforward application of KEe=hf−BE to find the ejected electron’s maximum kinetic energy, since BE is given.
Solution for (a)
Photon energy is given by
E=hf
Since we are given the wavelength rather than the frequency, we solve the familiar relationship c=fλ for the frequency, yielding
f=cλ.
Combining these two equations gives the useful relationship
E=hcλ.
Now substituting known values yields
E=(6.63×10−34 J⋅s)(3.00×108 m/s)420×10−9 m=4.74×10−19 J.
Converting to eV, the energy of the photon is
E=(4.74×10−19 J)1 eV1.6×10−19 J=2.96 eV.
Solution for (b)
Finding the kinetic energy of the ejected electron is now a simple application of the equation KEe=hf−BE. Substituting the photon energy and binding energy yields
KEe=hf−BE=2.96 eV−2.71 eV=0.246 eV.
Discussion
The energy of this 420-nm photon of violet light is a tiny fraction of a joule, and so it is no wonder that a single photon would be difficult for us to sense directly—humans are more attuned to energies on the order of joules. But looking at the energy in electron volts, we can see that this photon has enough energy to affect atoms and molecules. A DNA molecule can be broken with about 1 eV of energy, for example, and typical atomic and molecular energies are on the order of eV, so that the UV photon in this example could have biological effects. The ejected electron (called a photoelectron) has a rather low energy, and it would not travel far, except in a vacuum. The electron would be stopped by a retarding potential of but 0.26 eV. This simply means that the 420-nm photons with their 2.96-eV energy are not much above the frequency threshold. You can show for yourself that the threshold wavelength is 459 nm (blue light). This means that if calcium metal is used in a light meter, the meter will be insensitive to wavelengths longer than those of blue light.
Section Summary
- The photoelectric effect is the process in which EM radiation ejects electrons from a material.
- Einstein proposed photons to be quanta of EM radiation having energy E=hf, where f is the frequency of the radiation.
- All EM radiation is composed of photons. As Einstein explained, all characteristics of the photoelectric effect are due to the interaction of individual photons with individual electrons.
- The maximum kinetic energy KEe of ejected electrons (photoelectrons) is given by KEe=hf−BE, where hf is the photon energy and BE is the binding energy (or work function) of the electron to the particular material.
Glossary
- photoelectric effect
- the phenomenon whereby some materials eject electrons when light is shined on them
- photon
- a quantum, or particle, of electromagnetic radiation
- photon energy
- the amount of energy a photon has; E=hf
- work function
- the amount of energy necessary to eject an electron from a material; the binding energy in photoelectric effect


